
Which of the following has a giant covalent structure?
(a)- Iodine crystal
(b)- Solid $C{{O}_{2}}$
(c)- Silica
(d)- White phosphorus
Answer
561.6k+ views
Hint: Covalent structure is made by those compounds which have covalent bonds between the atoms. Mostly the elements of group 14 have the ability to form giant molecules, like diamond and graphite.
Complete step by step answer:
- Covalent structures are made by those compounds which have covalent bonds between the atoms and covalent bonds are made between the non-metal atoms. Mostly the elements of group 14 have the ability to form giant molecules, like diamond and graphite.
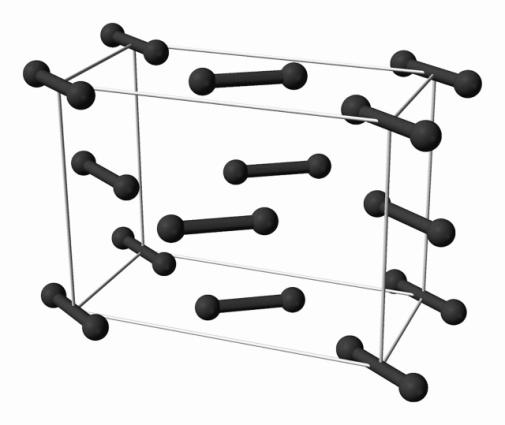
- The structure of iodine crystal is described as a face-centered-cubic structure, since the molecular formula of iodine is ${{I}_{2}}$, so they form a covalent bond between two iodine atoms only and they do not form giant molecules. The structure is given below:
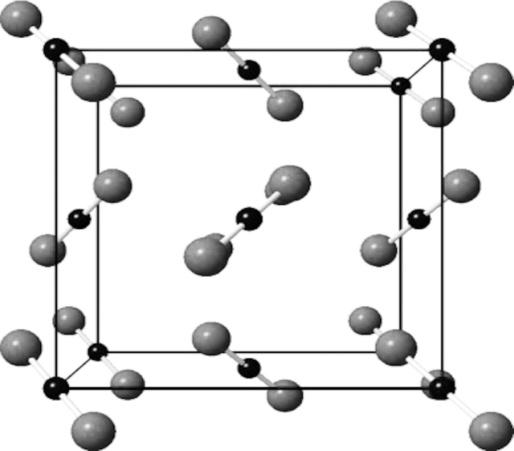
- The structure of solid $C{{O}_{2}}$ is also not a giant structure, because the carbon forms two double bonds with oxygen atoms and doesn't have valency to form another atom to form a giant structure. The structure is given below:
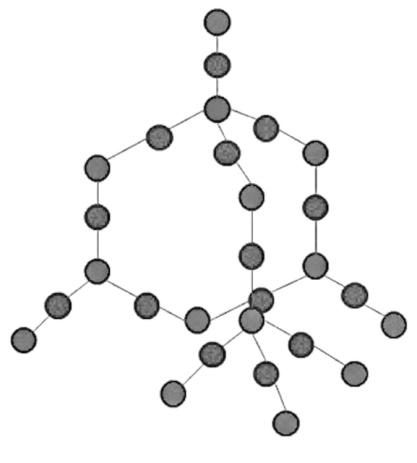
The structure of silica is a giant molecular structure because the formula of silica is $Si{{O}_{2}}$ and silicon usually forms four single covalent bonds, so two of the bonds are occupied by the oxygen atom and the other two are used to join with the oxygen atoms of other silica molecules. This leads to the formation of a giant network and its structure is given below:
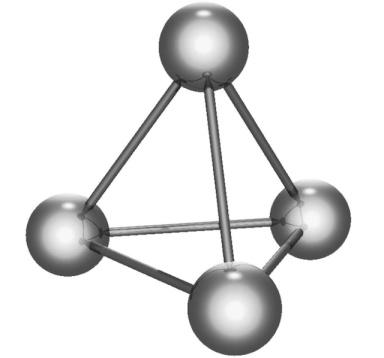
The white phosphorus has a tetrahedral structure in which there are four atoms joined by six covalent bonds, and it doesn’t form the giant molecular structure. The structure is given below:
The correct option is option “C” .
Note: The melting point of the giant covalent structures is very high because due to the interlocking structure the bonds are strong and need very high energy to break. This is due to the fact that diamond, graphite, silica have a very high melting point.
Complete step by step answer:
- Covalent structures are made by those compounds which have covalent bonds between the atoms and covalent bonds are made between the non-metal atoms. Mostly the elements of group 14 have the ability to form giant molecules, like diamond and graphite.
- The structure of iodine crystal is described as a face-centered-cubic structure, since the molecular formula of iodine is ${{I}_{2}}$, so they form a covalent bond between two iodine atoms only and they do not form giant molecules. The structure is given below:

- The structure of solid $C{{O}_{2}}$ is also not a giant structure, because the carbon forms two double bonds with oxygen atoms and doesn't have valency to form another atom to form a giant structure. The structure is given below:

The structure of silica is a giant molecular structure because the formula of silica is $Si{{O}_{2}}$ and silicon usually forms four single covalent bonds, so two of the bonds are occupied by the oxygen atom and the other two are used to join with the oxygen atoms of other silica molecules. This leads to the formation of a giant network and its structure is given below:

The white phosphorus has a tetrahedral structure in which there are four atoms joined by six covalent bonds, and it doesn’t form the giant molecular structure. The structure is given below:

The correct option is option “C” .
Note: The melting point of the giant covalent structures is very high because due to the interlocking structure the bonds are strong and need very high energy to break. This is due to the fact that diamond, graphite, silica have a very high melting point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




