
Which of the following has a plane of symmetry?
(A) 1,4-dimethylcyclohexene
(B) 1,3-dimethylcyclohexene
(C) trans-1,3-dimethylcyclohexane
(D) cis-1,2-dimethylcyclohexane
Answer
532.2k+ views
Hint: To solve this question we first need to know what is the plane of symmetry. An imaginary plane which bisects the molecule into two to give halves which are mirror images of each other is known as the plane of symmetry.
Complete answer:
Now, to determine which molecule has a plane of symmetry, we first need to draw the structure of each molecule.
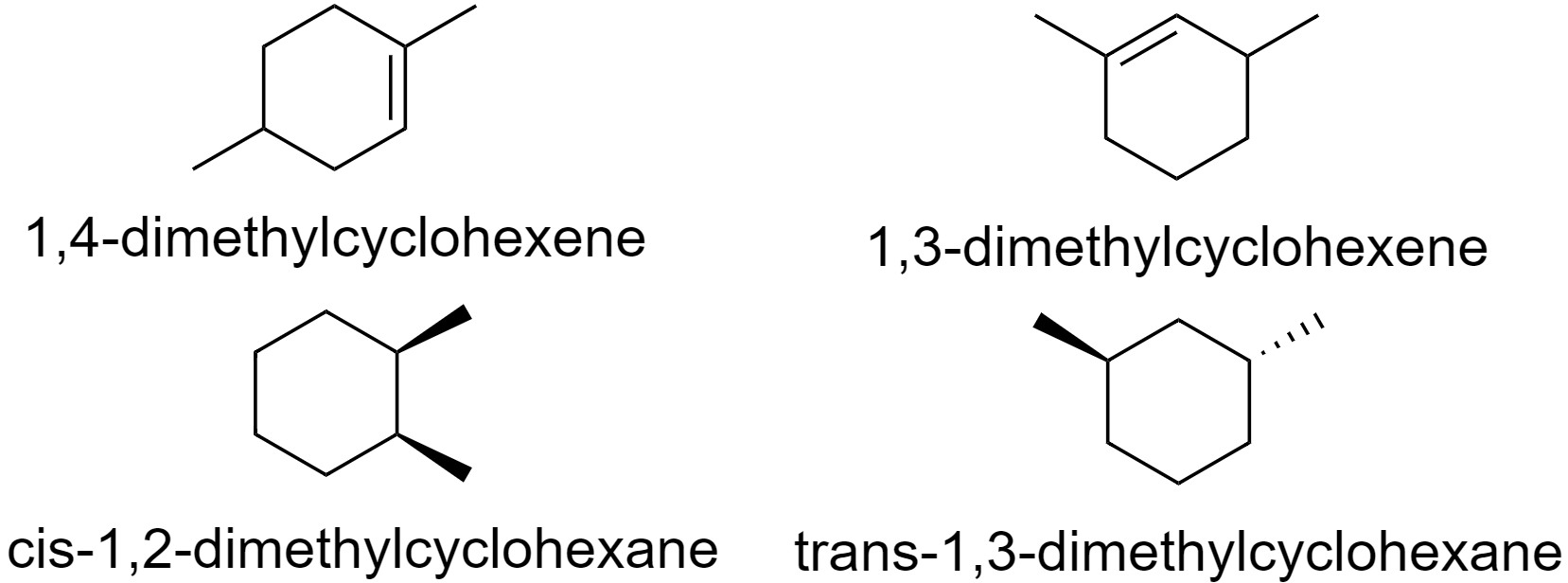
Now, from the structures above we can see that in 1,4-dimethylcyclohexene, 1,3-dimethylcyclohexene, and trans-1,3-dimethylcyclohexane, we cannot draw any axis that will bisect the molecule in such a way that it produces mirror images.
This is because, in 1,4-dimethylcyclohexene and 1,3-dimethylcyclohexene, the methyl groups are substituted at only one of the carbon atoms which is double bonded and hence does not have a plane of symmetry.
In trans-1,3-dimethylcyclohexane, the substituted methyl groups are on the opposite sides of the carbon atom plane hence they do not produce mirror images.
Whereas, in cis-1,2-dimethylcyclohexane, when the molecule is bisected horizontally, it divides into two mirror images.
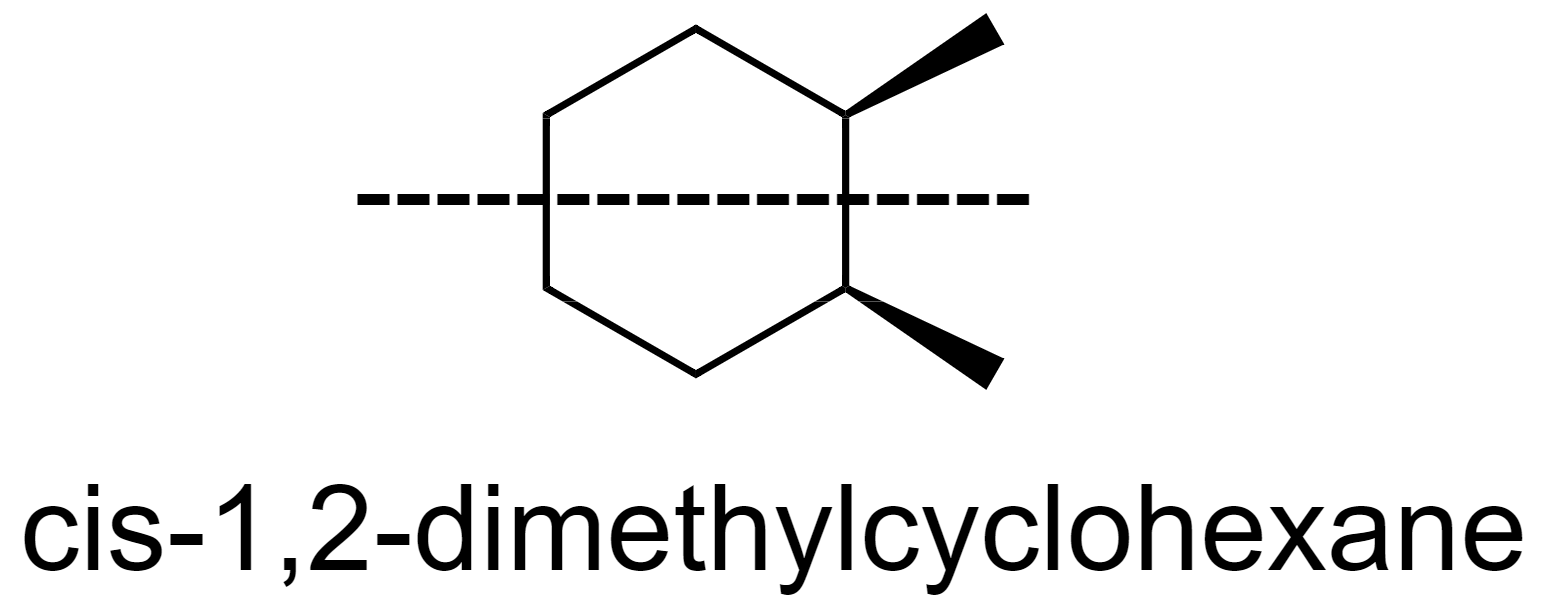
Hence the correct answer is option (D) cis-1,2-dimethylcyclohexane has a plane of symmetry.
Additional Information:
It should be noted that if a molecule has a plane of symmetry, it can be said that the molecule is achiral. An achiral molecule is superimposable on its mirror image.
Note:
It is easy to confuse the plane of symmetry of trans isomer and a cis isomer of a molecule.
One could assume that simply 1,2-dimethylcyclohexane has a plane of symmetry without knowing the cis-trans orientation of the molecule. Hence to determine the plane of symmetry of any molecule, one must know about the orientation of the molecule.
Complete answer:
Now, to determine which molecule has a plane of symmetry, we first need to draw the structure of each molecule.

Now, from the structures above we can see that in 1,4-dimethylcyclohexene, 1,3-dimethylcyclohexene, and trans-1,3-dimethylcyclohexane, we cannot draw any axis that will bisect the molecule in such a way that it produces mirror images.
This is because, in 1,4-dimethylcyclohexene and 1,3-dimethylcyclohexene, the methyl groups are substituted at only one of the carbon atoms which is double bonded and hence does not have a plane of symmetry.
In trans-1,3-dimethylcyclohexane, the substituted methyl groups are on the opposite sides of the carbon atom plane hence they do not produce mirror images.
Whereas, in cis-1,2-dimethylcyclohexane, when the molecule is bisected horizontally, it divides into two mirror images.

Hence the correct answer is option (D) cis-1,2-dimethylcyclohexane has a plane of symmetry.
Additional Information:
It should be noted that if a molecule has a plane of symmetry, it can be said that the molecule is achiral. An achiral molecule is superimposable on its mirror image.
Note:
It is easy to confuse the plane of symmetry of trans isomer and a cis isomer of a molecule.
One could assume that simply 1,2-dimethylcyclohexane has a plane of symmetry without knowing the cis-trans orientation of the molecule. Hence to determine the plane of symmetry of any molecule, one must know about the orientation of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




