
Which of the following has zero dipole moment?
A ) \[{\text{ClF}}\]
B ) \[{\text{PC}}{{\text{l}}_3}\]
C ) \[{\text{Si}}{{\text{F}}_4}\]
D ) \[{\text{CFC}}{{\text{l}}_3}\]
Answer
531k+ views
Hint: Based on the molecular geometry, determine if the individual bond dipoles cancels each other or not . If the individual bond dipoles cancels each other, the molecule is nonpolar, with zero dipole moment. If the individual bond dipoles do not cancel each other, the molecule is polar, with non-zero dipole moment.
Complete step by step answer:
\[{\text{ClF}}\] is a linear molecule as it has only two atoms. Two atoms are from two different elements having different electronegativities. Hence, the chlorine-fluorine bond is polar, and has a dipole moment. Hence, the molecule is a polar molecule and has a dipole moment.
In \[{\text{PC}}{{\text{l}}_3}\] molecule, the central phosphorus atom has three bond pairs of electrons and one lone pair of electrons. It is \[s{p^3}\] hybridised with trigonal pyramidal geometry. Phosphorus and chlorine atoms have different electronegativity. Hence, phosphorus chlorine bond is polar. The individual bond dipoles do not cancel each other. Hence, the molecule is polar with non zero dipole moment.
In \[{\text{Si}}{{\text{F}}_{\text{4}}}\] the central silicon atom has four silicon-fluorine single bonds. The number of lone pairs of electrons is zero. The central silicon atom has four bonding domains and zero lone pairs of electrons. Hence, the silicon atom is \[s{p^3}\] hybridised. The geometry around silicon atoms is tetrahedral. The individual bond dipoles cancel each other. Hence, it is a nonpolar molecule.
In \[{\text{CFC}}{{\text{l}}_3}\] the central carbon atom has three carbon-chlorine single bonds and one carbon-fluorine single bond. The number of lone pairs of electrons is zero. The central carbon atom has four bonding domains and zero lone pairs of electrons. Hence, the carbon atom is \[s{p^3}\] hybridised. The geometry around the carbon atom is tetrahedral. The electronegativity of carbon is different from that of fluorine or chlorine. Hence, carbon-chlorine and carbon-fluorine bonds are polar. The individual bond dipoles do not cancel each other. Hence, it is a polar molecule with non-zero dipole moment.
Thus, the molecule with zero dipole moment is silicon tetrafluoride \[{\text{Si}}{{\text{F}}_4}\].
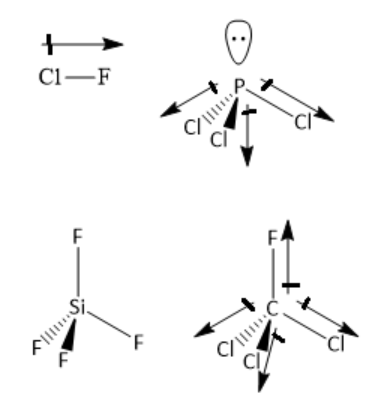
Hence, the correct option is the option C ) \[{\text{Si}}{{\text{F}}_4}\].
Note:
In heteronuclear diatomic / polyatomic molecules, polar bonds are present as atoms of different elements have different electronegativities. Not all molecules containing polar bonds have net dipole moment. There are some molecules containing polar bonds, yet these molecules have zero dipole moment. This is particularly true of symmetrical molecules in which individual bond dipoles cancel each other.
Complete step by step answer:
\[{\text{ClF}}\] is a linear molecule as it has only two atoms. Two atoms are from two different elements having different electronegativities. Hence, the chlorine-fluorine bond is polar, and has a dipole moment. Hence, the molecule is a polar molecule and has a dipole moment.
In \[{\text{PC}}{{\text{l}}_3}\] molecule, the central phosphorus atom has three bond pairs of electrons and one lone pair of electrons. It is \[s{p^3}\] hybridised with trigonal pyramidal geometry. Phosphorus and chlorine atoms have different electronegativity. Hence, phosphorus chlorine bond is polar. The individual bond dipoles do not cancel each other. Hence, the molecule is polar with non zero dipole moment.
In \[{\text{Si}}{{\text{F}}_{\text{4}}}\] the central silicon atom has four silicon-fluorine single bonds. The number of lone pairs of electrons is zero. The central silicon atom has four bonding domains and zero lone pairs of electrons. Hence, the silicon atom is \[s{p^3}\] hybridised. The geometry around silicon atoms is tetrahedral. The individual bond dipoles cancel each other. Hence, it is a nonpolar molecule.
In \[{\text{CFC}}{{\text{l}}_3}\] the central carbon atom has three carbon-chlorine single bonds and one carbon-fluorine single bond. The number of lone pairs of electrons is zero. The central carbon atom has four bonding domains and zero lone pairs of electrons. Hence, the carbon atom is \[s{p^3}\] hybridised. The geometry around the carbon atom is tetrahedral. The electronegativity of carbon is different from that of fluorine or chlorine. Hence, carbon-chlorine and carbon-fluorine bonds are polar. The individual bond dipoles do not cancel each other. Hence, it is a polar molecule with non-zero dipole moment.
Thus, the molecule with zero dipole moment is silicon tetrafluoride \[{\text{Si}}{{\text{F}}_4}\].

Hence, the correct option is the option C ) \[{\text{Si}}{{\text{F}}_4}\].
Note:
In heteronuclear diatomic / polyatomic molecules, polar bonds are present as atoms of different elements have different electronegativities. Not all molecules containing polar bonds have net dipole moment. There are some molecules containing polar bonds, yet these molecules have zero dipole moment. This is particularly true of symmetrical molecules in which individual bond dipoles cancel each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




