
Which of the following has zero dipole moment?
A. \[B{F_3}\]
B. BeClBr
C. \[C{H_2}C{l_2}\]
D. COS
Answer
562.8k+ views
Hint: The dipole moment is formed when there is electronegativity difference between the atoms. When the moment of dipole is toward the pair of electrons, then the dipole moment is larger.
Complete step by step answer:
A. \[B{F_3}\]: In boron trifluoride, the structure is trigonal planar geometry. The fluorine is the most electronegative atom, so the movement of dipole is towards the fluorine atom. As the magnitude is the same for the two B-F bonds which are opposite to the third B-F bond, it will cancel the dipole of the third B-F bond and as a result the dipole will be zero.
The structure of boron trifluoride is shown below.
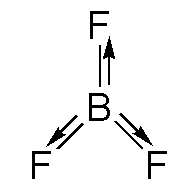
B. BeClBr
BeClBr forms a linear geometry as the two substituents attached are not same.
C. \[C{H_2}C{l_2}\]
In \[C{H_2}C{l_2}\], the chlorine atom is the most electronegative as compared to carbon and then hydrogen. The dipole moment will be towards the right side C-Cl and in vertical direct C-Cl. Both the dipoles will not be canceled out.
The structure is shown below.
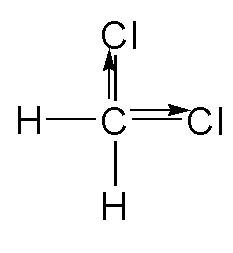
D. COS
The molecule COS has dipole as it is linear and asymmetric in nature. The oxygen atom is more electronegative than the Sulphur atom, so the oxygen will attract more electrons as compared to Sulphur. The two dipoles do not cancel out each other.
So, the correct answer is Option A.
Note: Dipole moment takes place when there is a separation of charge into positive ion and negative ion due to unequal attraction of electrons by the two atoms. The dipole moment helps to determine the polarity of the compound.
Complete step by step answer:
A. \[B{F_3}\]: In boron trifluoride, the structure is trigonal planar geometry. The fluorine is the most electronegative atom, so the movement of dipole is towards the fluorine atom. As the magnitude is the same for the two B-F bonds which are opposite to the third B-F bond, it will cancel the dipole of the third B-F bond and as a result the dipole will be zero.
The structure of boron trifluoride is shown below.

B. BeClBr
BeClBr forms a linear geometry as the two substituents attached are not same.
C. \[C{H_2}C{l_2}\]
In \[C{H_2}C{l_2}\], the chlorine atom is the most electronegative as compared to carbon and then hydrogen. The dipole moment will be towards the right side C-Cl and in vertical direct C-Cl. Both the dipoles will not be canceled out.
The structure is shown below.

D. COS
The molecule COS has dipole as it is linear and asymmetric in nature. The oxygen atom is more electronegative than the Sulphur atom, so the oxygen will attract more electrons as compared to Sulphur. The two dipoles do not cancel out each other.
So, the correct answer is Option A.
Note: Dipole moment takes place when there is a separation of charge into positive ion and negative ion due to unequal attraction of electrons by the two atoms. The dipole moment helps to determine the polarity of the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




