
Which of the following hexoses will form the same osazone when treated with excess phenylhydrazine?
A ) D-glucose, D-fructose and D-galactose
B ) D-glucose, D-fructose and D-mannose
C ) D-glucose, D-mannose and D-galactose
D ) D-fructose, D-mannose and D-galactose
Answer
592.5k+ views
Hint: Identify the similarities/ differences between configurations for first two carbon atoms for the given hexoses since phenyl hydrazine only affects the configuration of first two carbon atoms.
Complete step by step answer:
Write the structures of given hexoses as follows:
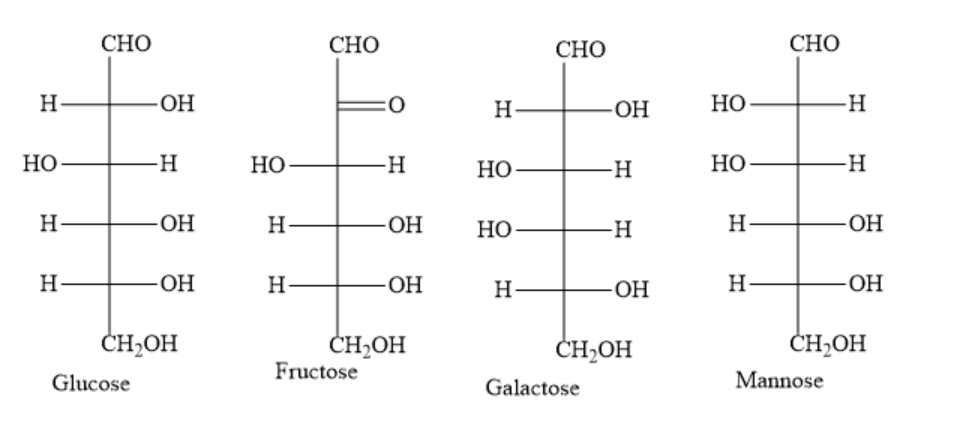
During the formation of osazone, only the first two carbon atoms participate. The remaining four carbon atoms remain as such.
\[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\] have different configurations only at second carbon atom. For the remaining carbon atoms, they have the same configuration.
In the Fischer projection formula for D-glucose, the hydroxyl group of the second carbon atom is on the right hand side. In the Fischer projection formula for D-mannose, the hydroxyl group of the second carbon atom is on the left hand side. D-fructose has a carbonyl group on the second carbon atom.
\[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\] form the same osazone when treated with excess phenylhydrazine.
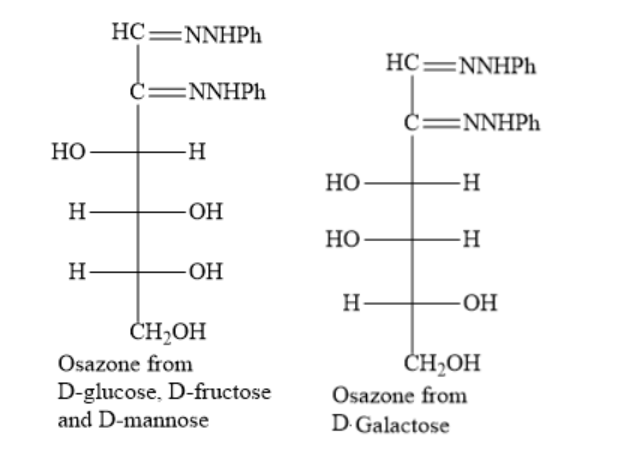
The structures of the osazone from \[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\]and D-galactose are as shown below:
Hence, the option B) \[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\] is the correct option.
Note:
When two reactions are added, the values of the enthalpy changes are also added. When two reactions are subtracted, the values of the enthalpy changes are also subtracted. When a reaction is divided with a number, the enthalpy change value is also divided with the same number.
Complete step by step answer:
Write the structures of given hexoses as follows:

During the formation of osazone, only the first two carbon atoms participate. The remaining four carbon atoms remain as such.
\[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\] have different configurations only at second carbon atom. For the remaining carbon atoms, they have the same configuration.
In the Fischer projection formula for D-glucose, the hydroxyl group of the second carbon atom is on the right hand side. In the Fischer projection formula for D-mannose, the hydroxyl group of the second carbon atom is on the left hand side. D-fructose has a carbonyl group on the second carbon atom.
\[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\] form the same osazone when treated with excess phenylhydrazine.
The structures of the osazone from \[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\]and D-galactose are as shown below:

Hence, the option B) \[D-glucose,\text{ }D-fructose\text{ }and\text{ }D-mannose\] is the correct option.
Note:
When two reactions are added, the values of the enthalpy changes are also added. When two reactions are subtracted, the values of the enthalpy changes are also subtracted. When a reaction is divided with a number, the enthalpy change value is also divided with the same number.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




