
Which of the following is a cyclic oxoacid?
(A) $ {H_4}{P_2}{O_7} $
(B) $ {H_4}{P_2}{O_6} $
(C) $ {H_3}{P_3}{O_9} $
(D) $ {H_5}{P_5}{O_{15}} $
Answer
517.8k+ views
Hint :Oxoacids (also known as oxyacids) are acids that contain oxygen. An oxoacid, to be more specific, is an acid that contains oxygen and contains at least one additional component that has one or more hydrogen atoms bound to oxygen. And cyclic oxoacids is an oxoacid whose chemical structure is cyclic.
Complete Step By Step Answer:
Now, to analyse the structure of the compounds first we need to make the chemical structures of all the compounds in the option.
Option (A) $ {H_4}{P_2}{O_7} $

Since, the structure is a chain and it's not cyclic. Hence, option (A) is incorrect.
Option (B) $ {H_4}{P_2}{O_6} $
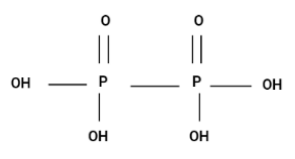
Since, the structure is a chain and it's not cyclic. Hence, option (B) is also incorrect.
Option (C) $ {H_3}{P_3}{O_9} $
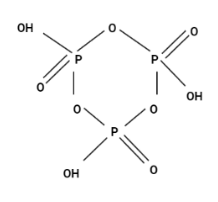
This structure is cyclic oxoacid. Hence, the correct option is (C).
Option (D) $ {H_5}{P_5}{O_{15}} $

The structure is a chain and it’s not cyclic. This structure is the same as option (C) , the only difference is that this structure is chain instead of cyclic. Hence, option (D) is also incorrect.
Therefore the correct option is (C).
Note :
Oxoacids are acids that contain oxygen as a component. Phosphorus is known to shape a variety of oxoacids, including $ {H_3}P{O_4} $ , $ {H_3}P{O_3} $ , and others. It is tetrahedrally surrounded by other atoms in phosphorus oxoacids.
Complete Step By Step Answer:
Now, to analyse the structure of the compounds first we need to make the chemical structures of all the compounds in the option.
Option (A) $ {H_4}{P_2}{O_7} $

Since, the structure is a chain and it's not cyclic. Hence, option (A) is incorrect.
Option (B) $ {H_4}{P_2}{O_6} $

Since, the structure is a chain and it's not cyclic. Hence, option (B) is also incorrect.
Option (C) $ {H_3}{P_3}{O_9} $

This structure is cyclic oxoacid. Hence, the correct option is (C).
Option (D) $ {H_5}{P_5}{O_{15}} $

The structure is a chain and it’s not cyclic. This structure is the same as option (C) , the only difference is that this structure is chain instead of cyclic. Hence, option (D) is also incorrect.
Therefore the correct option is (C).
Note :
Oxoacids are acids that contain oxygen as a component. Phosphorus is known to shape a variety of oxoacids, including $ {H_3}P{O_4} $ , $ {H_3}P{O_3} $ , and others. It is tetrahedrally surrounded by other atoms in phosphorus oxoacids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




