
Which of the following is a primary halide?
(a) Iso-propyl iodide
(b) sec-butyl iodide
(c) tert-butyl bromide
(d) neo-hexyl chloride
Answer
569.1k+ views
Hint: Primary halide is that in which the carbon which is linked to the halogen consists of only one alkyl group and by drawing the structures of the given compounds, we can easily identify the primary halide. Now solve it.
Complete answer:
First of all ,let’s discuss what is alkyl halide. The alkyl halide consists of the alkane and the halogens i.e. Cl, Br , F etc. It is formed when alkanes react with the halogens in the presence of light. Alkyl hides are also known as the haloalkanes.
Now coming to the primary halide. If the carbon atom attached to the halogen consists of one alkyl group, then the alkyl halide is known as the primary halide.
Now coming next to the statement;
We will first draw the structure of the given organic compounds , then we can easily identify the primary halide.
(a) Iso-propyl iodide:- The structure of iso-propyl iodide is as;
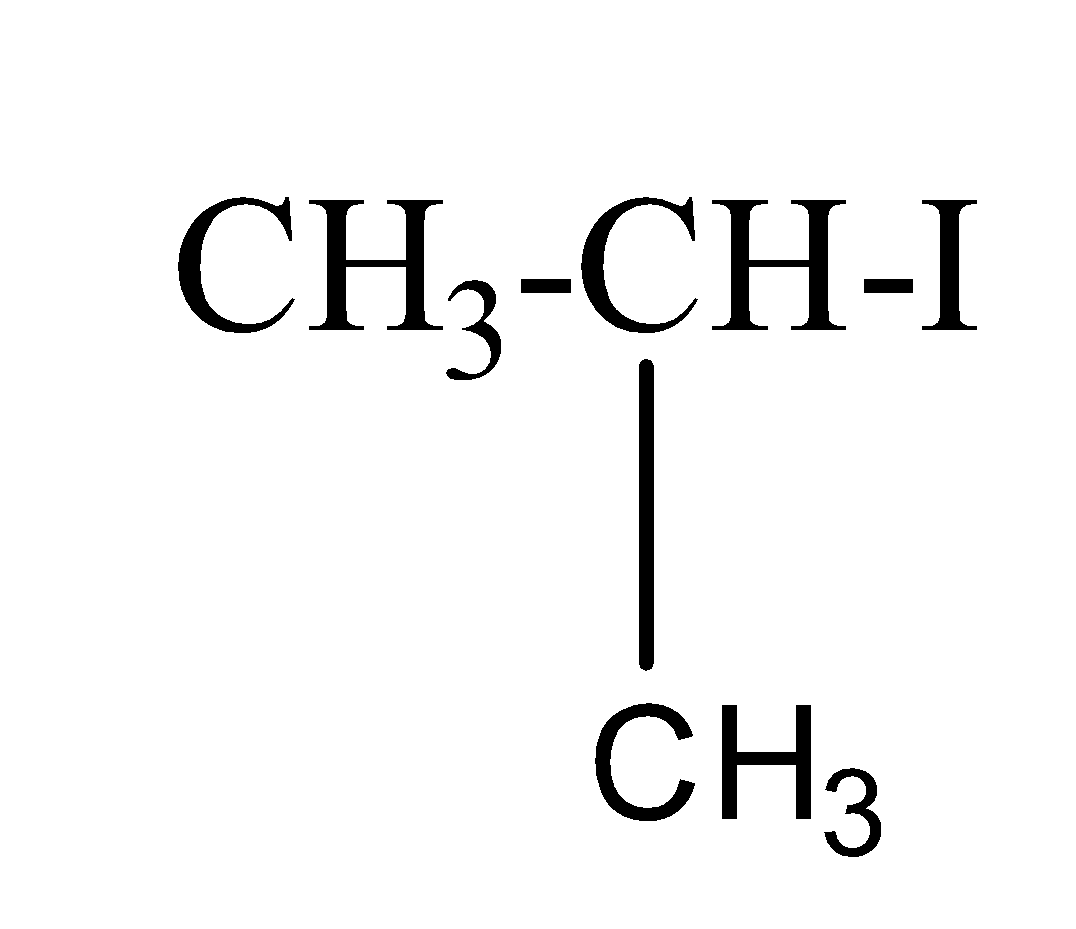
(b) sec-butyl iodide:- The structure of sec-butyl iodide is as;
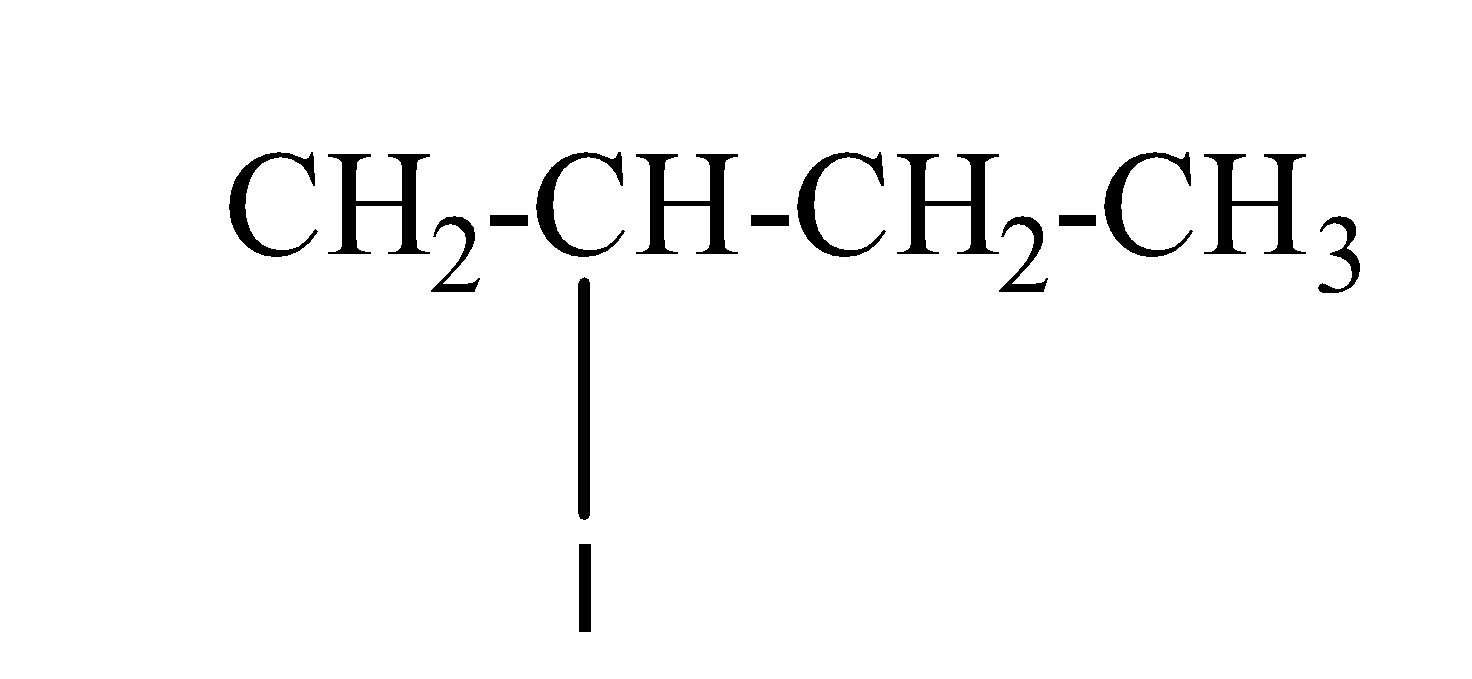
(C) tert-butyl bromide:- The structure of tert-butyl bromide is as;
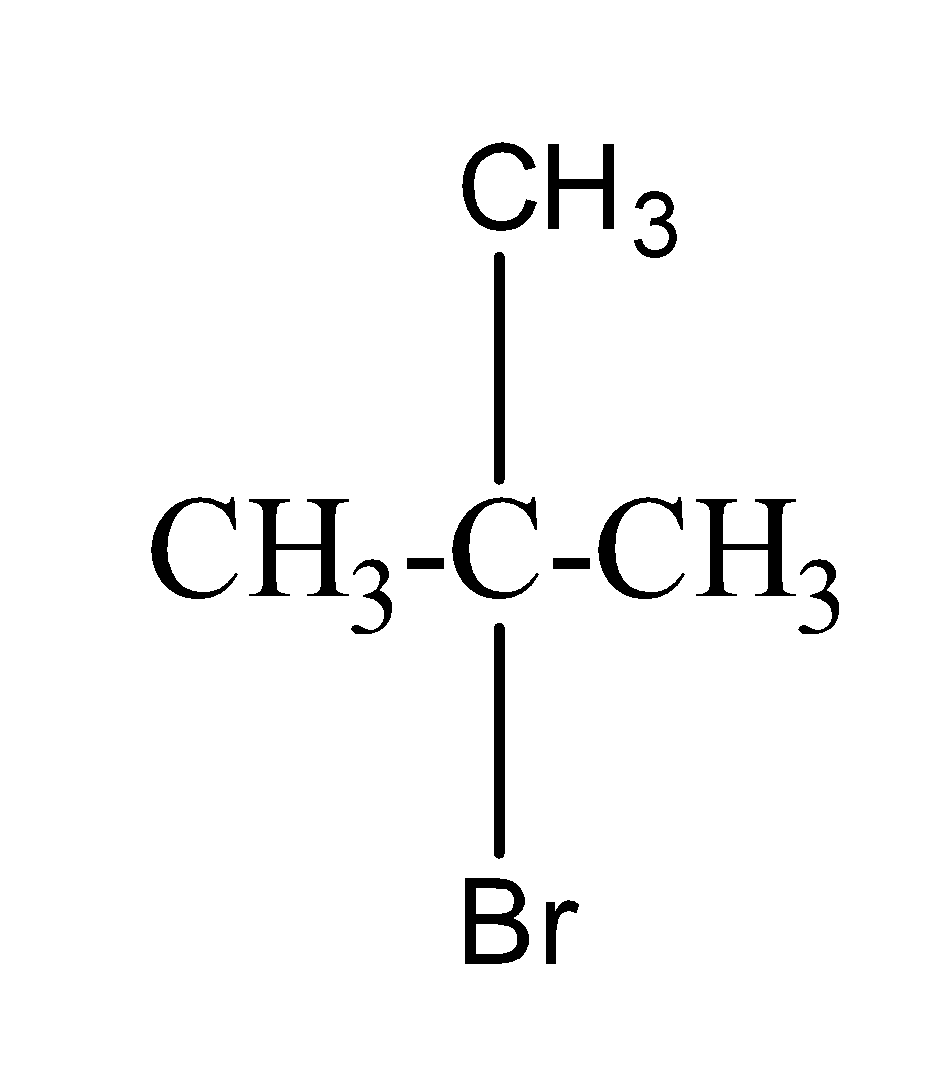
(d) neo-hexyl chloride:- The structure of neo-hexyl chloride is as;
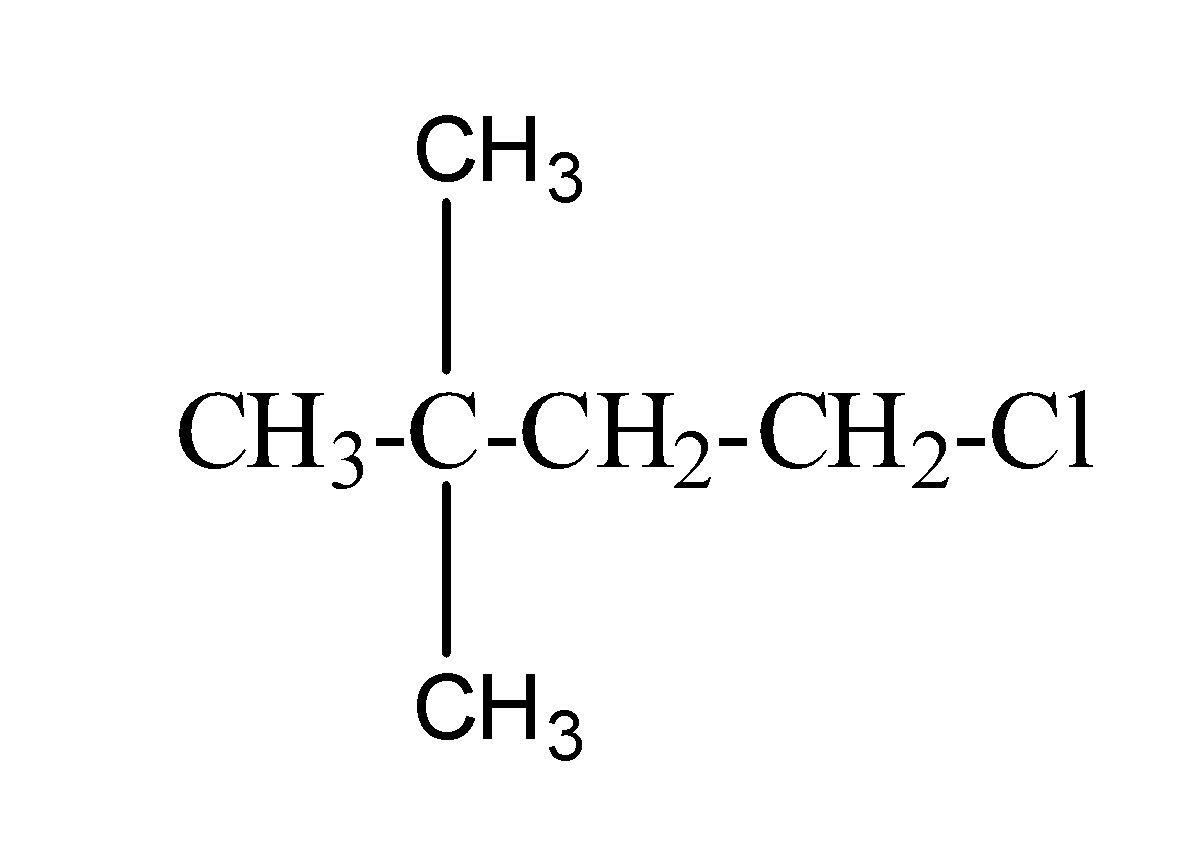
So, thus, from the above it is clear that neo-hexyl chloride is the primary halide.
Hence, option (d) is correct.
Note:
If the carbon atom attached to the halogen consists of two alkyl groups, then the alkyl halide is known as the secondary halide.
And If the carbon atom attached to the halogen consists of three alkyl groups, then the alkyl halide is known as the tertiary halide.
Complete answer:
First of all ,let’s discuss what is alkyl halide. The alkyl halide consists of the alkane and the halogens i.e. Cl, Br , F etc. It is formed when alkanes react with the halogens in the presence of light. Alkyl hides are also known as the haloalkanes.
Now coming to the primary halide. If the carbon atom attached to the halogen consists of one alkyl group, then the alkyl halide is known as the primary halide.
Now coming next to the statement;
We will first draw the structure of the given organic compounds , then we can easily identify the primary halide.
(a) Iso-propyl iodide:- The structure of iso-propyl iodide is as;

(b) sec-butyl iodide:- The structure of sec-butyl iodide is as;
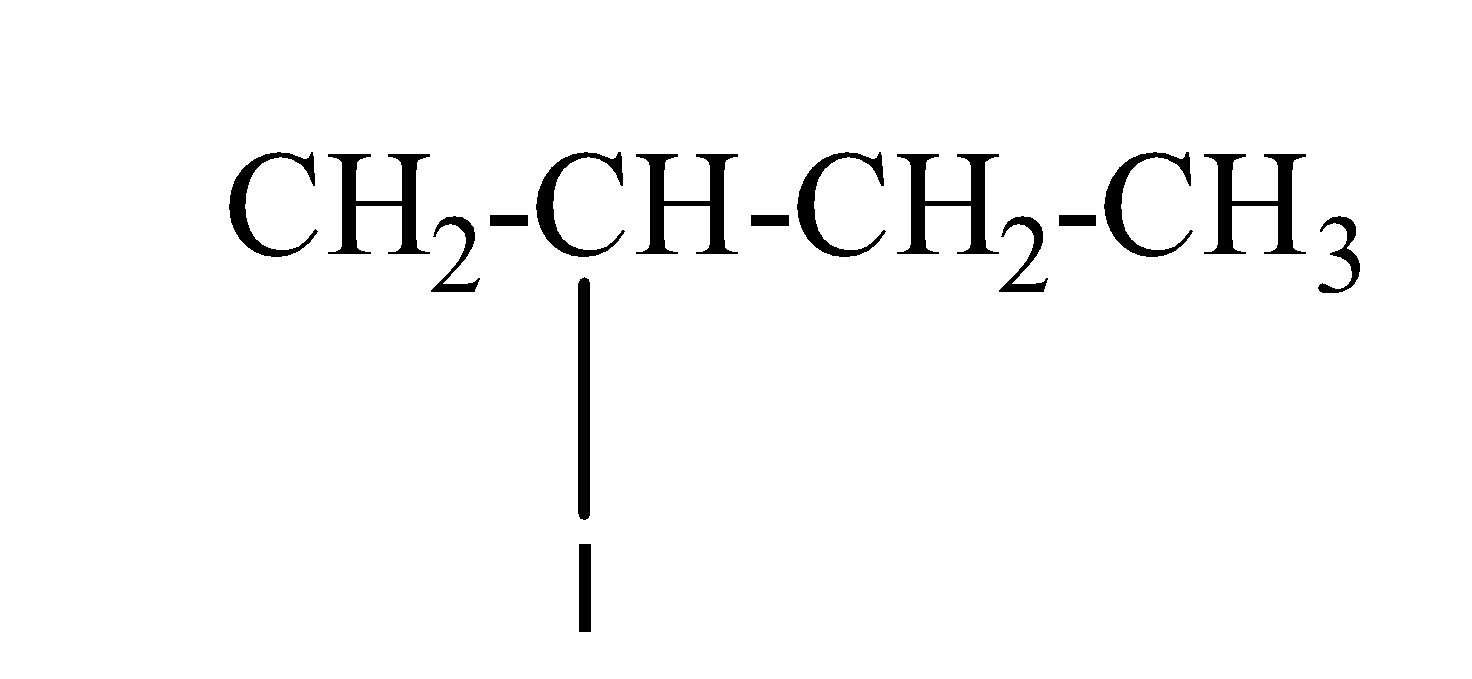
(C) tert-butyl bromide:- The structure of tert-butyl bromide is as;

(d) neo-hexyl chloride:- The structure of neo-hexyl chloride is as;

So, thus, from the above it is clear that neo-hexyl chloride is the primary halide.
Hence, option (d) is correct.
Note:
If the carbon atom attached to the halogen consists of two alkyl groups, then the alkyl halide is known as the secondary halide.
And If the carbon atom attached to the halogen consists of three alkyl groups, then the alkyl halide is known as the tertiary halide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




