
Which of the following is a weak acid
(A) \[C{H_3}COOH\]
(B) \[HCl\]
(C) \[{H_2}S{O_4}\]
(D) \[HN{O_3}\]
Answer
506.7k+ views
Hint: There are many theories developed till now to explain the acid-base concept of different acids and bases present. Different theories are Bronsted-Lowry theory of acid and base and the lewis acid- base theory.
Complete answer:
So we will discuss the Bronsted-Lowry theory to understand the acid and base phenomenon for the better perspective of the question.
So according to Bronsted-Lowry theory, a Bronsted-Lowry acid is the chemical species which donates the hydrogen ion and the Bronsted-Lowry base is the chemical species that accepts the hydrogen ion.
So, now we have a basic idea regarding the acid and base species.
Moving forward the strong and weak acids are characterised on the basis of their dissociation in the water. I.e. their ability to give hydrogen ions or undergo dissociation.
And as among the given options in the question we can see that the acetic acid does not dissociate properly in the water, it undergoes only partial dissociation.
Let’s look at the dissociation reaction of the acetic acid:
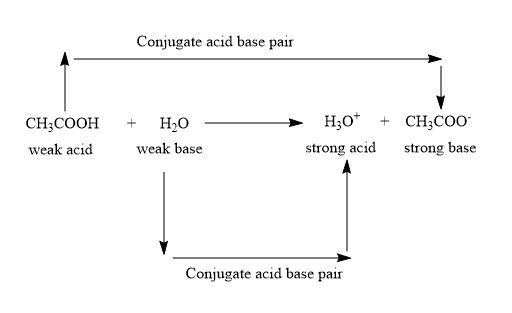
And all the other given acids i.e. hydrochloric acid, sulphuric acid and nitric acid dissociate completely in water and are the strong acids.
So the correct answer to the question is option A. i.e. acetic acid, \[C{H_3}COOH\] .
Note:
So, acetic acid being a mild acid is frequently used in daily life in the form of vinegar, which is an important compound in the food industries. It is actually a colourless liquid with a very strong pungent smell which is its characteristic.
Complete answer:
So we will discuss the Bronsted-Lowry theory to understand the acid and base phenomenon for the better perspective of the question.
So according to Bronsted-Lowry theory, a Bronsted-Lowry acid is the chemical species which donates the hydrogen ion and the Bronsted-Lowry base is the chemical species that accepts the hydrogen ion.
So, now we have a basic idea regarding the acid and base species.
Moving forward the strong and weak acids are characterised on the basis of their dissociation in the water. I.e. their ability to give hydrogen ions or undergo dissociation.
And as among the given options in the question we can see that the acetic acid does not dissociate properly in the water, it undergoes only partial dissociation.
Let’s look at the dissociation reaction of the acetic acid:

And all the other given acids i.e. hydrochloric acid, sulphuric acid and nitric acid dissociate completely in water and are the strong acids.
So the correct answer to the question is option A. i.e. acetic acid, \[C{H_3}COOH\] .
Note:
So, acetic acid being a mild acid is frequently used in daily life in the form of vinegar, which is an important compound in the food industries. It is actually a colourless liquid with a very strong pungent smell which is its characteristic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




