
Which of the following is an antiaromatic compound?
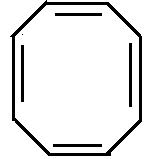
A.
B.
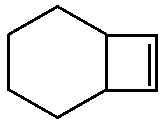
C.
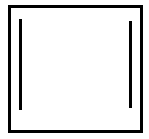
D.




Answer
547.2k+ views
Hint: We need to find the anti- aromatic compound. For solving this we use the aromaticity rule. First, the compound to be observed must be cyclic. Also, every atom in the ring must be conjugated. Then the molecule must have $ \left( {4n + 2} \right)\pi $ electrons. Anti-aromatic compounds have 4n pi electrons.
Complete step by step answer:
We will start by mentioning the aromaticity rule.
This rule is followed by aromatic and anti- aromatic compounds.
First, the compound to be observed must be cyclic. Also, every atom in the ring must be conjugated. Then the molecule must have $ \left( {4n + 2} \right)\pi $ electrons. At last, the molecule must be flat, but there are rare exceptions.
Option A is cyclooctatriene which is a non-aromatic compound. It changes to tub shaped structure.
Option B is cyclobutadiene which has $ {\text{2 \times n}}{{\text{e}}^{\text{ - }}}{\text{ where n = 2 = 4}}{{\text{e}}^{\text{ - }}} $ ,so it is antiaromatic.
Option C is not planar as the two middle C atoms are $ s{p^3} $ hybridised, thus making it non planar.
Option D is pentagon with negative charge and it is aromatic as it has $ {\text{2n}}{{\text{e}}^{\text{ - }}}{\text{ where n = 3}} $ .
Now we need to select the correct option.
Thus, the correct option is B.
Note:
Aromatic compounds are chemical compounds that consist of conjugated planar ring systems accompanied by delocalized pi-electron clouds in place of individual alternating double and single bonds. They are also called aromatics or arenes. The best examples are toluene and benzene.
Antiaromatic compounds are not necessarily unstable - they are just less stable than a bunch of ethenes connected by sigma bonds.
Another method to approach these types of questions are:
A molecule is aromatic if it is cyclic, planar, completely conjugated compound with $ 4n + 2\pi $ electrons. It is antiaromatic if all of this is correct except it has 4n electrons, any deviation from these criteria makes it non-aromatic.
Complete step by step answer:
We will start by mentioning the aromaticity rule.
This rule is followed by aromatic and anti- aromatic compounds.
First, the compound to be observed must be cyclic. Also, every atom in the ring must be conjugated. Then the molecule must have $ \left( {4n + 2} \right)\pi $ electrons. At last, the molecule must be flat, but there are rare exceptions.
Option A is cyclooctatriene which is a non-aromatic compound. It changes to tub shaped structure.
Option B is cyclobutadiene which has $ {\text{2 \times n}}{{\text{e}}^{\text{ - }}}{\text{ where n = 2 = 4}}{{\text{e}}^{\text{ - }}} $ ,so it is antiaromatic.
Option C is not planar as the two middle C atoms are $ s{p^3} $ hybridised, thus making it non planar.
Option D is pentagon with negative charge and it is aromatic as it has $ {\text{2n}}{{\text{e}}^{\text{ - }}}{\text{ where n = 3}} $ .
Now we need to select the correct option.
Thus, the correct option is B.
Note:
Aromatic compounds are chemical compounds that consist of conjugated planar ring systems accompanied by delocalized pi-electron clouds in place of individual alternating double and single bonds. They are also called aromatics or arenes. The best examples are toluene and benzene.
Antiaromatic compounds are not necessarily unstable - they are just less stable than a bunch of ethenes connected by sigma bonds.
Another method to approach these types of questions are:
A molecule is aromatic if it is cyclic, planar, completely conjugated compound with $ 4n + 2\pi $ electrons. It is antiaromatic if all of this is correct except it has 4n electrons, any deviation from these criteria makes it non-aromatic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




