
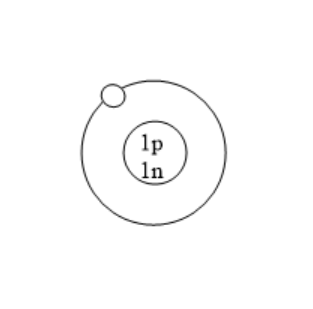
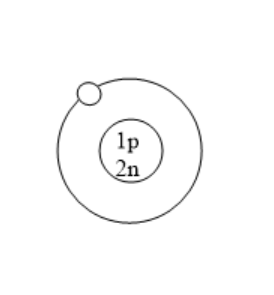
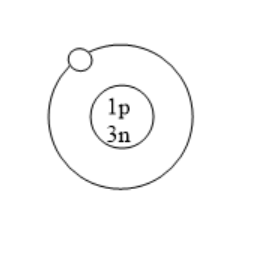
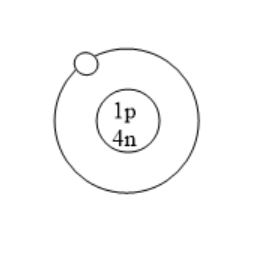
Which of the following is an atom of tritium?
(A)
(B)
(C)
(D)




Answer
524.4k+ views
Hint: The element Hydrogen has three isotopes. Isotopes are different versions of an element which have different numbers of neutrons. The isotopes of hydrogen are protium, Deuterium and tritium. Hydrogen has an atomic number of one. Every isotope of hydrogen has one proton. The mass number of protium is one. The mass number of deuterium is two. The mass number of tritium is three.
Complete step by step answer:
All the isotopes of hydrogen have one proton but different number of neutrons. Protium has zero neutrons. Deuterium has one neutron. Tritium has two neutrons. Tritium is used in nuclear weapons. It is also used to control nuclear fusion. It can be used as an energy source in future and is also used in self powered lighting. Tritium has two neutrons because it has one proton and mass number is three.
Therefore, option B is correct.

Note: Before solving problems, remember that for atoms with no charge, the number of protons is always equal to the atomic number and number of electrons is also equal to atomic number. Number of neutrons can be calculated using the difference of mass number and atomic number. Here, in this problem we know that the atomic number of tritium is one as it is an isotope of hydrogen. The mass number of deuterium is three. So, the number of neutrons is the difference of mass number and atomic number that is $3 - 1 = 2$. Therefore, the number of neutrons in tritium are two. Tritium consists of one proton and two neutrons.
Complete step by step answer:
All the isotopes of hydrogen have one proton but different number of neutrons. Protium has zero neutrons. Deuterium has one neutron. Tritium has two neutrons. Tritium is used in nuclear weapons. It is also used to control nuclear fusion. It can be used as an energy source in future and is also used in self powered lighting. Tritium has two neutrons because it has one proton and mass number is three.
Therefore, option B is correct.

Note: Before solving problems, remember that for atoms with no charge, the number of protons is always equal to the atomic number and number of electrons is also equal to atomic number. Number of neutrons can be calculated using the difference of mass number and atomic number. Here, in this problem we know that the atomic number of tritium is one as it is an isotope of hydrogen. The mass number of deuterium is three. So, the number of neutrons is the difference of mass number and atomic number that is $3 - 1 = 2$. Therefore, the number of neutrons in tritium are two. Tritium consists of one proton and two neutrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




