
Which of the following is an example of ring chain isomerism?
A. 2-Methyl pentane and 3-methyl pentane
B. Ethanal and ethanone
C. Cyclopentane and methylcyclobutane
D. Cyclopentane and pentane
Answer
611.7k+ views
Hint: The compounds which have the same molecular formula but show two different structures one is in open chain form and other is in cyclic form are called ring chain isomers.
Complete answer:
The phenomenon of existence of two or more compounds having the same chemical formula but different chemical structures is known as isomerism, they differ in properties and the arrangements of atoms therefore they are known as isomers.
In ring chain isomerism the compound can transform itself into both, open chain structure into a ring structure.
For example propene and cyclopropane both having the same molecular formula i.e. ${{\text{C}}_3}{{\text{H}}_6}$and show open chain and a ring structure as:
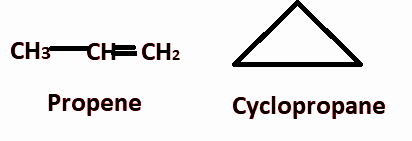
In above examples
2-Methyl pentane and 3-methyl pentane both show same molecular formula ${{\text{C}}_6}{{\text{H}}_{14}}$ but do not show ring like structure therefore they are not ring chain isomers.
Ethanal has molecular formula ${{\text{C}}_2}{{\text{H}}_4}{\text{O}}$ and ethanone has molecular formula as ${{\text{C}}_6}{{\text{H}}_8}{\text{O}}$ both have different molecular formula therefore they are not ring chain isomers.
Cyclopentane and methyl cyclobutane both having the same molecular formula i.e. ${{\text{C}}_5}{{\text{H}}_{10}}$ but do not show open chain structure therefore they are not ring chain isomers.
Cyclopentane has molecular formula ${{\text{C}}_5}{{\text{H}}_{10}}$ and pentane has molecular formula ${{\text{C}}_5}{{\text{H}}_{12}}$ both have different molecular formula therefore they are not ring chain isomers.
Therefore none of the above given examples is of ring chain isomerism.
Hence none of the above options is correct.
Note: Isomers are broadly divided in two categories one is constitutional and other is stereoisomer whereas constitutional isomers differ in bonding and connectivity while stereoisomers differ in three dimension orientation.
Complete answer:
The phenomenon of existence of two or more compounds having the same chemical formula but different chemical structures is known as isomerism, they differ in properties and the arrangements of atoms therefore they are known as isomers.
In ring chain isomerism the compound can transform itself into both, open chain structure into a ring structure.
For example propene and cyclopropane both having the same molecular formula i.e. ${{\text{C}}_3}{{\text{H}}_6}$and show open chain and a ring structure as:

In above examples
2-Methyl pentane and 3-methyl pentane both show same molecular formula ${{\text{C}}_6}{{\text{H}}_{14}}$ but do not show ring like structure therefore they are not ring chain isomers.
Ethanal has molecular formula ${{\text{C}}_2}{{\text{H}}_4}{\text{O}}$ and ethanone has molecular formula as ${{\text{C}}_6}{{\text{H}}_8}{\text{O}}$ both have different molecular formula therefore they are not ring chain isomers.
Cyclopentane and methyl cyclobutane both having the same molecular formula i.e. ${{\text{C}}_5}{{\text{H}}_{10}}$ but do not show open chain structure therefore they are not ring chain isomers.
Cyclopentane has molecular formula ${{\text{C}}_5}{{\text{H}}_{10}}$ and pentane has molecular formula ${{\text{C}}_5}{{\text{H}}_{12}}$ both have different molecular formula therefore they are not ring chain isomers.
Therefore none of the above given examples is of ring chain isomerism.
Hence none of the above options is correct.
Note: Isomers are broadly divided in two categories one is constitutional and other is stereoisomer whereas constitutional isomers differ in bonding and connectivity while stereoisomers differ in three dimension orientation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




