
Which of the following is an isomer of ethanol?
A. Methanol
B. Dimethyl ether
C. Diethyl ether
D. Ethylene glycol
Answer
600.3k+ views
Hint: To answer this question we should know that isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. So, we have to find that option which has the same molecular formula as ethanol.
Step by step answer:
We should know that ethanol is a simple alcohol with the chemical formula ${{C}_{2}}{{H}_{6}}O$ or ${{C}_{2}}{{H}_{5}}OH$ (an ethyl group linked to a hydroxyl group), and is often observed as ${{C}_{2}}{{H}_{6}}O$. Ethanol is a volatile, flammable, colourless liquid with a slight characteristic odour. It is a psychoactive substance and is the principal active ingredient found in alcoholic drinks.
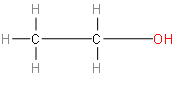
Above represented is the structure of ethanol.
So, according to the definition of isomer they are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule. If you can make an apparently different molecule just by rotating single bonds, it's not different - it's still the same molecule.
So, we have to find that option which has the same structure as ethanol.
By taking the first option, we have methanol. Methanol has a chemical formula $C{{H}_{3}}OH$. And its structural formula will be as follows:
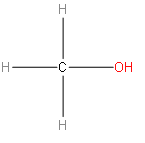
The above structure doesn’t look like an isomer of ethanol.
Now, we take the second option dimethyl ether. Then we should know that, it is the organic compound with the formula $C{{H}_{3}}OC{{H}_{3}}$, simplified to ${{C}_{2}}{{H}_{6}}O$. This looks the same with ethanol.
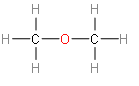
This dimethyl looks like a structural isomer of ethanol. Now we should take a quick look at the structures of other two options.
Third option is diethyl ether. It has a formula of ${{\left( {{C}_{2}}{{H}_{5}} \right)}_{2}}O$.
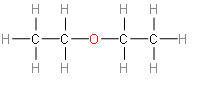
This option doesn’t look like any isomer of ethanol.
Option D is Ethylene glycol. It is an organic compound with the formula ${{\left( C{{H}_{2}}OH \right)}_{2}}$.
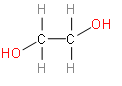
This option also doesn’t look like an isomer structure of ethanol.
From the above discussion and by observing each and every option we came to know that the answer of this question is option B. Dimethyl ether and ethanol are functional isomers. In functional isomers key groups of atoms are arranged in a particular way. The best example is this question. In this question both of the compound dimethyl ether and ethanol have ${{C}_{2}}{{H}_{6}}O$ as molecular formula.
Note: We should know the difference between ethanol and dimethyl ether. The key difference between ethanol and dimethyl ether is that the ethanol is a colourless liquid at room temperature which has high volatility whereas dimethyl ether is a colourless gas at room temperature. We should note that ethanol is highly flammable; thus, it is used as a fuel as well. Moreover, it is a highly volatile compound. We should note that dimethyl ether is a nonpolar compound. This means dimethyl ether has no polarity. That is because of its symmetrical molecular structure. Therefore, it is a good solvent for nonpolar compounds. However, it is chemically unreactive when compared with other organic compounds.
Step by step answer:
We should know that ethanol is a simple alcohol with the chemical formula ${{C}_{2}}{{H}_{6}}O$ or ${{C}_{2}}{{H}_{5}}OH$ (an ethyl group linked to a hydroxyl group), and is often observed as ${{C}_{2}}{{H}_{6}}O$. Ethanol is a volatile, flammable, colourless liquid with a slight characteristic odour. It is a psychoactive substance and is the principal active ingredient found in alcoholic drinks.

Above represented is the structure of ethanol.
So, according to the definition of isomer they are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule. If you can make an apparently different molecule just by rotating single bonds, it's not different - it's still the same molecule.
So, we have to find that option which has the same structure as ethanol.
By taking the first option, we have methanol. Methanol has a chemical formula $C{{H}_{3}}OH$. And its structural formula will be as follows:

The above structure doesn’t look like an isomer of ethanol.
Now, we take the second option dimethyl ether. Then we should know that, it is the organic compound with the formula $C{{H}_{3}}OC{{H}_{3}}$, simplified to ${{C}_{2}}{{H}_{6}}O$. This looks the same with ethanol.

This dimethyl looks like a structural isomer of ethanol. Now we should take a quick look at the structures of other two options.
Third option is diethyl ether. It has a formula of ${{\left( {{C}_{2}}{{H}_{5}} \right)}_{2}}O$.

This option doesn’t look like any isomer of ethanol.
Option D is Ethylene glycol. It is an organic compound with the formula ${{\left( C{{H}_{2}}OH \right)}_{2}}$.

This option also doesn’t look like an isomer structure of ethanol.
From the above discussion and by observing each and every option we came to know that the answer of this question is option B. Dimethyl ether and ethanol are functional isomers. In functional isomers key groups of atoms are arranged in a particular way. The best example is this question. In this question both of the compound dimethyl ether and ethanol have ${{C}_{2}}{{H}_{6}}O$ as molecular formula.
Note: We should know the difference between ethanol and dimethyl ether. The key difference between ethanol and dimethyl ether is that the ethanol is a colourless liquid at room temperature which has high volatility whereas dimethyl ether is a colourless gas at room temperature. We should note that ethanol is highly flammable; thus, it is used as a fuel as well. Moreover, it is a highly volatile compound. We should note that dimethyl ether is a nonpolar compound. This means dimethyl ether has no polarity. That is because of its symmetrical molecular structure. Therefore, it is a good solvent for nonpolar compounds. However, it is chemically unreactive when compared with other organic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




