
Which of the following is aromatic in nature?




Answer
478.5k+ views
Hint: The aromatic compounds are those which have one more ring in its structure and it contains pi electrons which are delocalized all over the structure. Also, the aromaticity of a compound can be found with the help of Huckel’s rule. According to which a compound which contains \[\left( {4n + 2} \right)\] pi electrons is considered in nature. Thus we will analyze each compound.
Formula Used:
For aromatic compounds, the number of pi electrons must be \[\left( {4n + 2} \right)\], where n is an integer starting from zero.
Complete Answer:
Aromatic compounds are those compounds that contain one more ring and follow the Huckel rule of aromaticity. According to the Huckel rule of aromaticity, a planar ring molecule will be aromatic in nature when it contains a number of pi electrons in order of \[\left( {4n + 2} \right)\]. We will analyze each compound respectively. Each double bond constitutes two electrons and a negative charge also indicates two electrons.
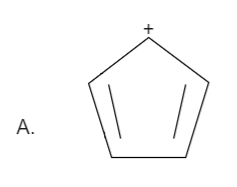
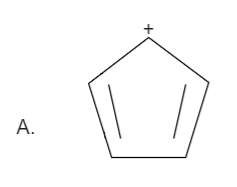
Here the total number of electrons are \[\left( {2 + 2 = 4} \right)\]. Thus it is not in order of \[\left( {4n + 2} \right)\], therefore it is not an aromatic compound.
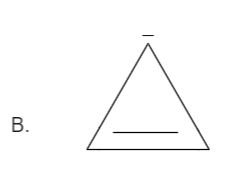
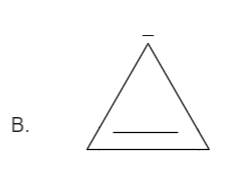
Total number pi bond is one and it has a negative charge also which in total has \[\left( {2 + 2 = 4} \right)\] electrons. Thus it is not in order of \[\left( {4n + 2} \right)\], therefore it is not an aromatic compound.
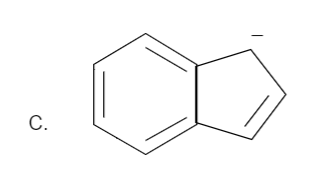
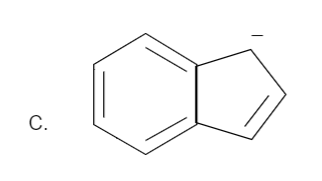
Total number of pi bonds is four and it has a negative charge also. Therefore the total number of electrons is: \[n = 2\] which is in the order of \[\left( {4n + 2} \right)\] for \[n = 2\]. Hence it is an aromatic compound.
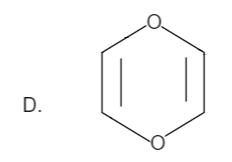
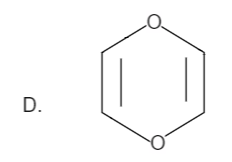
Here the total number of electrons are \[\left( {2 + 2 = 4} \right)\]. Thus it is not in order of \[\left( {4n + 2} \right)\], therefore it is not an aromatic compound.
Hence the correct option is C.
Note:
It must be noted that each pi bond constitutes two electrons and a negative charge accounts for two electrons where a positive charge does not account for any electron. This is because a negative charge is developed when atoms accept electrons and a positive charge is developed when atoms lose electrons. Also for aromatic compounds, the compound must be planar.
Formula Used:
For aromatic compounds, the number of pi electrons must be \[\left( {4n + 2} \right)\], where n is an integer starting from zero.
Complete Answer:
Aromatic compounds are those compounds that contain one more ring and follow the Huckel rule of aromaticity. According to the Huckel rule of aromaticity, a planar ring molecule will be aromatic in nature when it contains a number of pi electrons in order of \[\left( {4n + 2} \right)\]. We will analyze each compound respectively. Each double bond constitutes two electrons and a negative charge also indicates two electrons.

Here the total number of electrons are \[\left( {2 + 2 = 4} \right)\]. Thus it is not in order of \[\left( {4n + 2} \right)\], therefore it is not an aromatic compound.

Total number pi bond is one and it has a negative charge also which in total has \[\left( {2 + 2 = 4} \right)\] electrons. Thus it is not in order of \[\left( {4n + 2} \right)\], therefore it is not an aromatic compound.

Total number of pi bonds is four and it has a negative charge also. Therefore the total number of electrons is: \[n = 2\] which is in the order of \[\left( {4n + 2} \right)\] for \[n = 2\]. Hence it is an aromatic compound.

Here the total number of electrons are \[\left( {2 + 2 = 4} \right)\]. Thus it is not in order of \[\left( {4n + 2} \right)\], therefore it is not an aromatic compound.
Hence the correct option is C.
Note:
It must be noted that each pi bond constitutes two electrons and a negative charge accounts for two electrons where a positive charge does not account for any electron. This is because a negative charge is developed when atoms accept electrons and a positive charge is developed when atoms lose electrons. Also for aromatic compounds, the compound must be planar.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




