
Which of the following is NOT a lewis acid?
A. $B2H6$
B. $BeCl2$
C. $AlH3$
D. $NH3$
Answer
581.7k+ views
Hint: Lewis acids are lone pair acceptors or we can say electron deficient compounds. Lewis bases are lone pair donor or electron rich compounds.
Complete solution:
Let us first discuss the structure and type of bonding in these compounds.
${B_2}H_6$ This compound is known as diborane .It is an electron deficient compound.In its structure, the four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bridging hydrogen atoms.
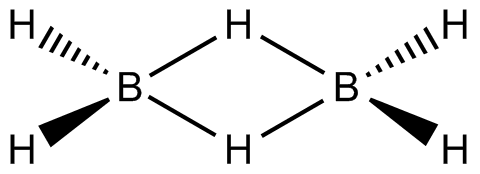
Since boron is electron deficient and this compound get stability using back bonding.So it accept a lone pair very easily and that is why it is a lewis acid
$BeCl_2$ Beryllium chloride is an electron deficient compound because octet of Be is not complete and it can easily accept a lone pair. So it is a lewis acid.

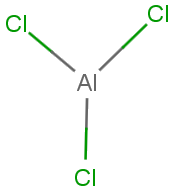
$AlC{l_3}$ It is an electron deficient compound molecule that has a tendency to accept a pair of electrons to achieve stable electronic configuration and thus behaves as lewis acids. It achieves stability by forming a dimer.
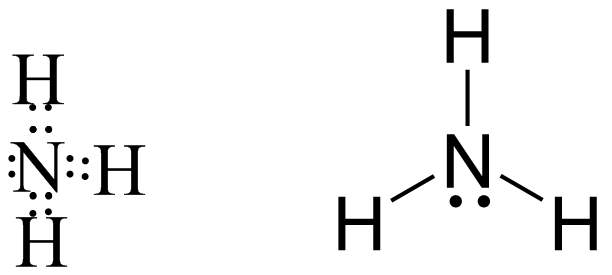
$N{H_3}$ It has pyramidal structure and has lone pairs on nitrogen atoms so it is an electron rich compound.It acts as a lewis base and can easily donate a lone pair of electrons.
Our correct option: D
Note:
Try to draw the structure of compound and lone pair on the compound. If compounds have lone pairs then it is not a lewis acid. For a compound to be a Lewis acid it should be capable of accepting electrons.
Complete solution:
Let us first discuss the structure and type of bonding in these compounds.
${B_2}H_6$ This compound is known as diborane .It is an electron deficient compound.In its structure, the four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bridging hydrogen atoms.

Since boron is electron deficient and this compound get stability using back bonding.So it accept a lone pair very easily and that is why it is a lewis acid
$BeCl_2$ Beryllium chloride is an electron deficient compound because octet of Be is not complete and it can easily accept a lone pair. So it is a lewis acid.

$AlC{l_3}$ It is an electron deficient compound molecule that has a tendency to accept a pair of electrons to achieve stable electronic configuration and thus behaves as lewis acids. It achieves stability by forming a dimer.

$N{H_3}$ It has pyramidal structure and has lone pairs on nitrogen atoms so it is an electron rich compound.It acts as a lewis base and can easily donate a lone pair of electrons.

Our correct option: D
Note:
Try to draw the structure of compound and lone pair on the compound. If compounds have lone pairs then it is not a lewis acid. For a compound to be a Lewis acid it should be capable of accepting electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




