
Which of the following is NOT a property of red phosphorus?
(A) Insoluble in carbon disulphide
(B) It does not show chemiluminescence by action of air
(C) It forms phosphine when treated with hot sodium hydroxide solution
(D) It is non-poisonous
Answer
524.1k+ views
Hint: Red phosphorus is an allotrope of the element phosphorus. It is also a derivative of ${P_4}$ molecule. As it is deep red in colour it is called red phosphorus. It has a powdery texture. It exists in amorphous form in nature. It has a melting point of $860K$. It is odourless and is solid at standard temperature and pressure.
Complete step by step answer:
The structure of red phosphorus resembles that of ${P_4}$ molecule. The structure of red phosphorus is given below:
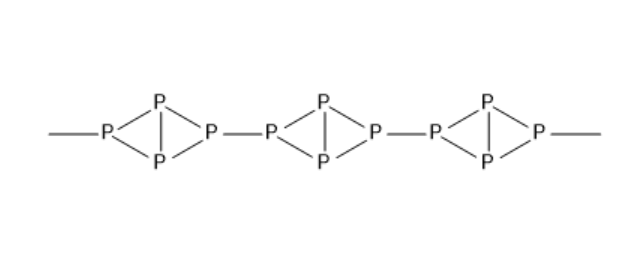
Red phosphorus is not soluble in water, alkalies and carbon disulphide because it exists in a polymeric structure. Under normal conditions, it remains steady. So, it does not show chemiluminescence. When white phosphorus is in contact with sodium hydroxide and water, it gives phosphine and also sodium hypophosphite. But red phosphorus does not give phosphine. Red phosphorus is considered non-toxic.
Therefore, option C is correct.
Note: Notice the word not in question carefully. Many students think that the question is property of red phosphorus and they choose option A. The structure of compounds helps in determining properties. So, notice that red phosphorus has a polymeric structure in which four phosphorus atoms together form a tetrahedral structure. Red phosphorus is the intermediate state between white phosphorus and violet phosphorus.
Complete step by step answer:
The structure of red phosphorus resembles that of ${P_4}$ molecule. The structure of red phosphorus is given below:

Red phosphorus is not soluble in water, alkalies and carbon disulphide because it exists in a polymeric structure. Under normal conditions, it remains steady. So, it does not show chemiluminescence. When white phosphorus is in contact with sodium hydroxide and water, it gives phosphine and also sodium hypophosphite. But red phosphorus does not give phosphine. Red phosphorus is considered non-toxic.
Therefore, option C is correct.
Note: Notice the word not in question carefully. Many students think that the question is property of red phosphorus and they choose option A. The structure of compounds helps in determining properties. So, notice that red phosphorus has a polymeric structure in which four phosphorus atoms together form a tetrahedral structure. Red phosphorus is the intermediate state between white phosphorus and violet phosphorus.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




