
Which of the following is not aromatic?
A.Benzene
B.cyclooctatetraenyl dianion
C.tropylium ion
D.cyclopentadienyl cation
Answer
582.3k+ views
Hint: For a molecule to be aromatic, it should be cyclic, planar, must fulfil Huckel’s rule and must be conjugated. His rule states that if a cyclic, planar molecule has 4n+2 π electrons, it is considered To be aromatic.
Complete step by step answer:
Non-aromatic does not follow Huckel’s rule. are highly unstable and highly reactive.
A.Benzene is cyclic, planar and has 6 $\pi $ electrons thus fulfilling Huckel’s rule. It is therefore aromatic.

6 $\pi $ electrons
B.Cyclooctatetraenyl Dianion has 10 $\pi $ electrons thus fulfilling Huckel’s rule. It is cyclic as well as planar. It is therefore aromatic.
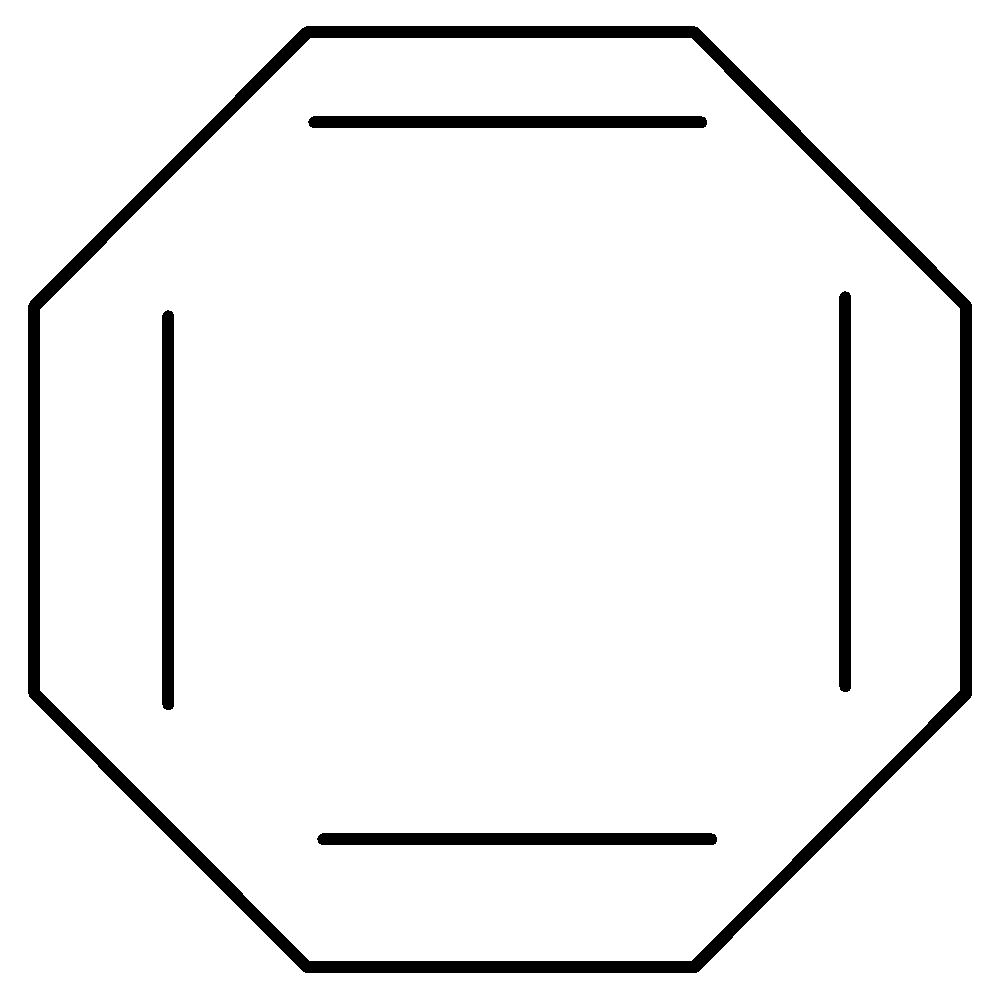
10 $\pi $ electrons
C.Tropylium Ion has 6 $\pi $ electrons. Thus it also fulfils Huckel’s rule. It is cyclic as well as planar. Therefore, it is also aromatic.
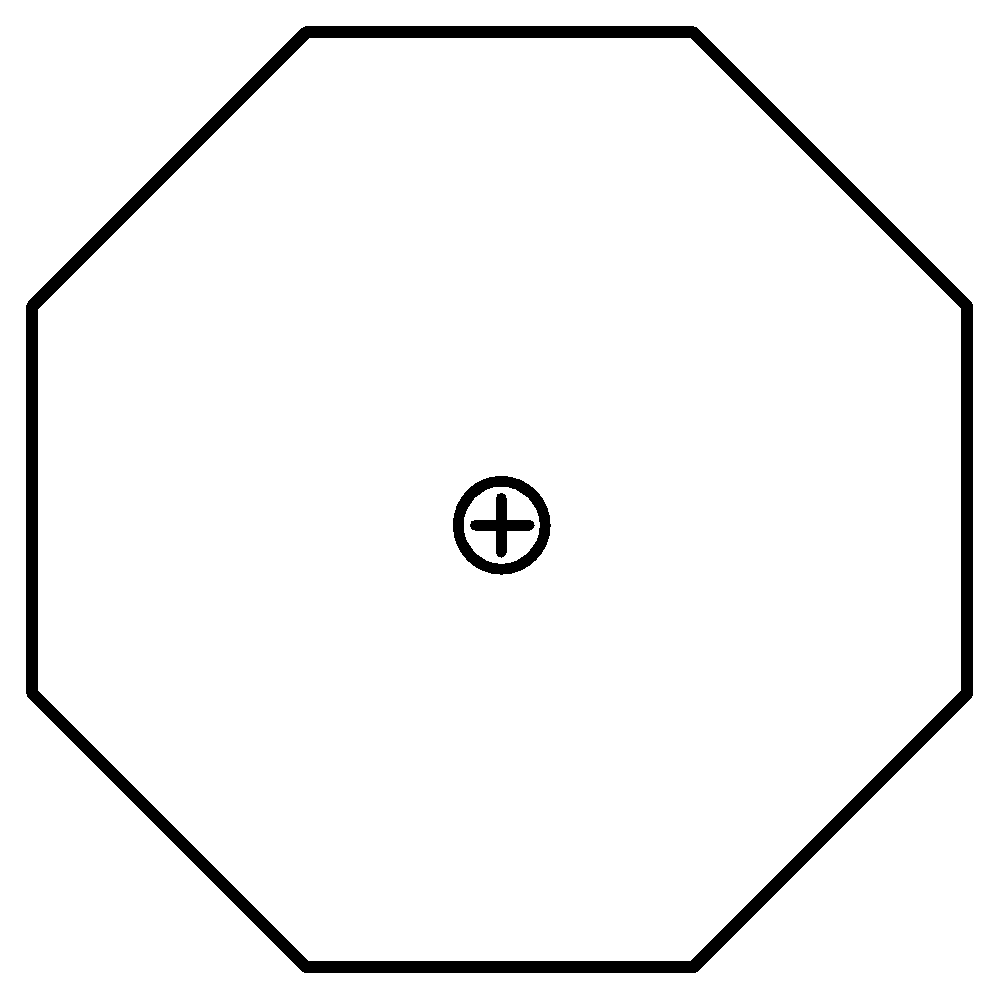
6 $\pi $ electrons
D.In Cyclopentadienyl cation, if a hydride anion \[\left( {H - } \right)\] is removed from the
\[sp3\] hybridized ring carbon, the cyclopentadienyl cation is formed, and hybridization of \[sp3\] ring carbon is altered to \[sp2\] .
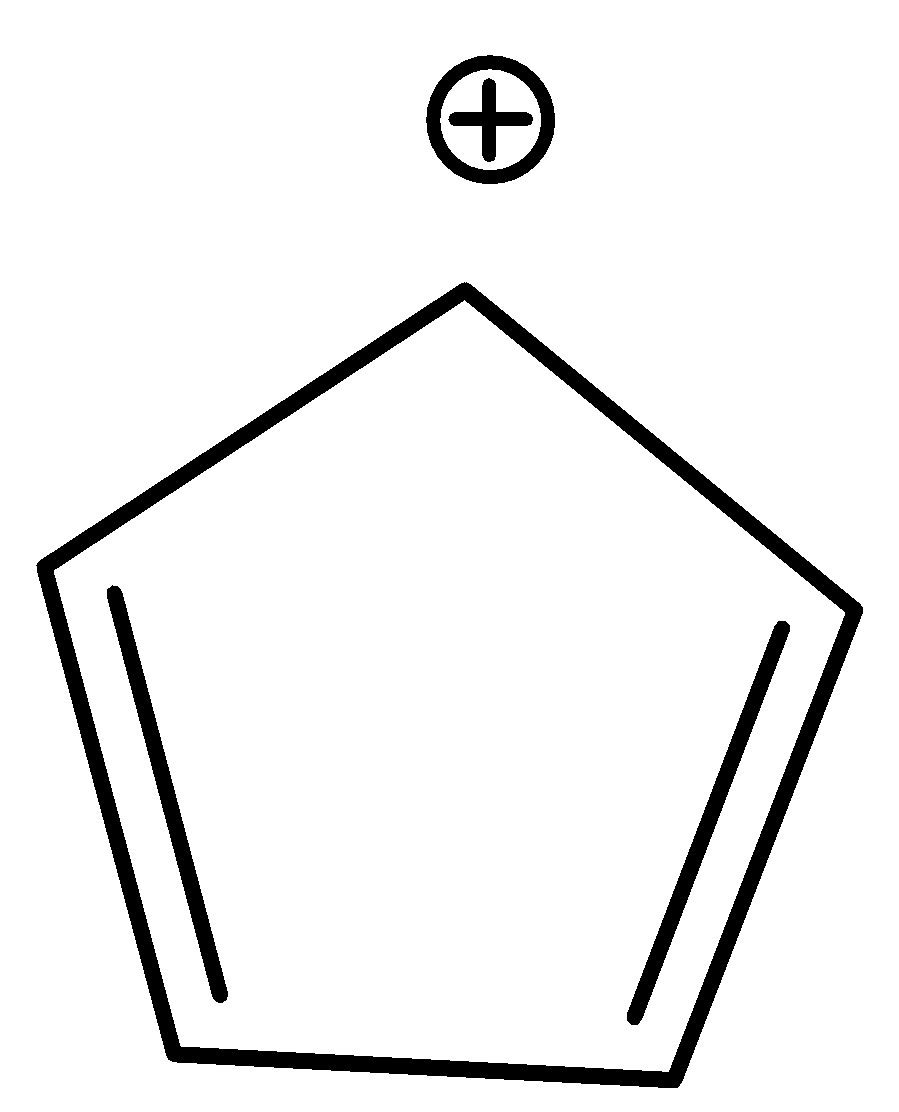
4 $\pi $ electrons
Thus, the cyclic cyclopentadienyl cation is planar and possesses a cyclic uninterrupted $\pi $ electron cloud. However, it does not follow Huckel’s rule as it has 4 $\pi $ electrons in a conjugated system. Thus, it is antiaromatic.
Hence option D is correct.
Note:
Molecules may change shape, becoming non-planar to avoid the instability of anti-aromaticity and therefore breaking some of the π interactions.
Complete step by step answer:
Non-aromatic does not follow Huckel’s rule. are highly unstable and highly reactive.
A.Benzene is cyclic, planar and has 6 $\pi $ electrons thus fulfilling Huckel’s rule. It is therefore aromatic.

6 $\pi $ electrons
B.Cyclooctatetraenyl Dianion has 10 $\pi $ electrons thus fulfilling Huckel’s rule. It is cyclic as well as planar. It is therefore aromatic.

10 $\pi $ electrons
C.Tropylium Ion has 6 $\pi $ electrons. Thus it also fulfils Huckel’s rule. It is cyclic as well as planar. Therefore, it is also aromatic.

6 $\pi $ electrons
D.In Cyclopentadienyl cation, if a hydride anion \[\left( {H - } \right)\] is removed from the
\[sp3\] hybridized ring carbon, the cyclopentadienyl cation is formed, and hybridization of \[sp3\] ring carbon is altered to \[sp2\] .

4 $\pi $ electrons
Thus, the cyclic cyclopentadienyl cation is planar and possesses a cyclic uninterrupted $\pi $ electron cloud. However, it does not follow Huckel’s rule as it has 4 $\pi $ electrons in a conjugated system. Thus, it is antiaromatic.
Hence option D is correct.
Note:
Molecules may change shape, becoming non-planar to avoid the instability of anti-aromaticity and therefore breaking some of the π interactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




