
Which of the following is the least effective method for preparing methyl cyclohexane via Wurtz reaction?
A.
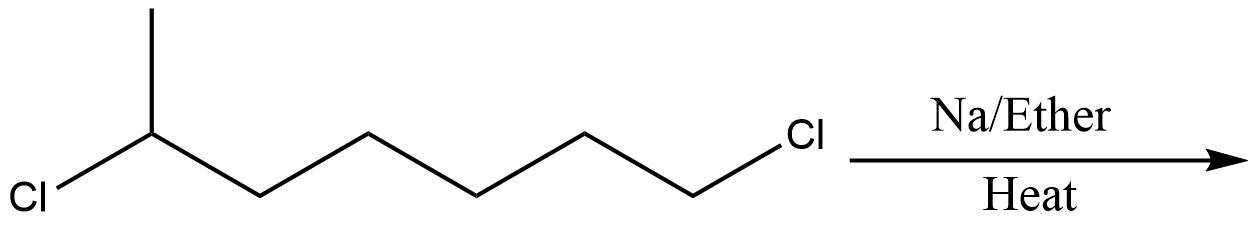
B.
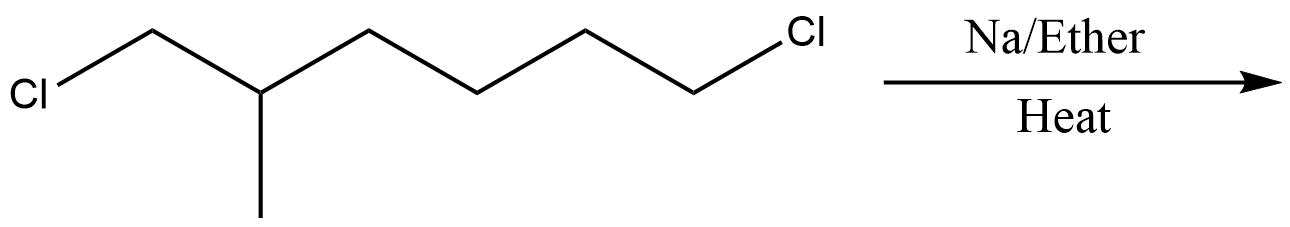
C.
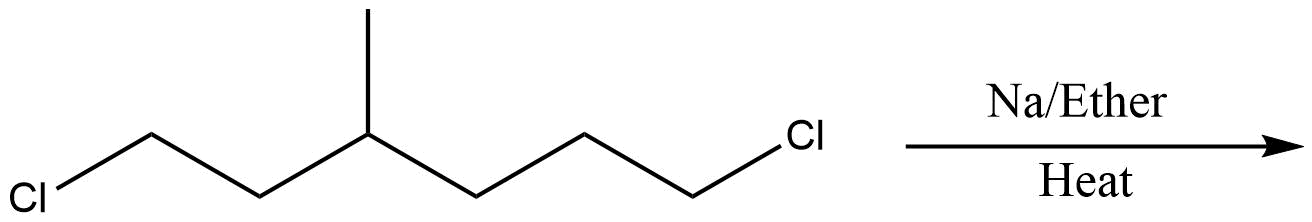
D.
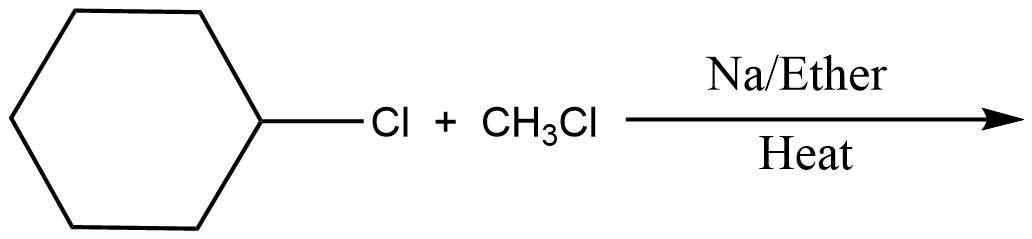



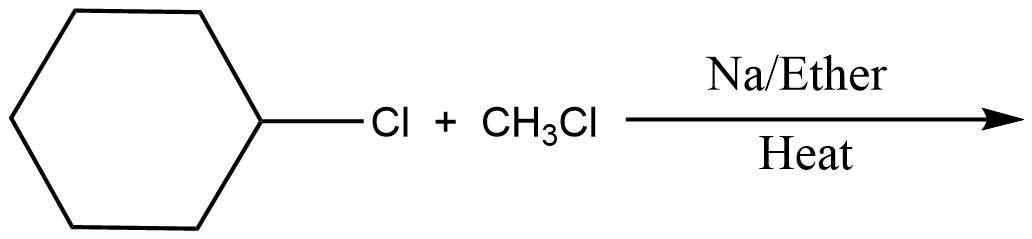
Answer
583.2k+ views
Hint: Wurtz reaction is an organic reaction that is used to couple two alkyl halides to form an alkane using sodium metal. Only primary alkyl halides can be used in this reaction. The reaction will be eliminated in the case of secondary or tertiary alkyl halides.
Complete step by step answer:
Wurtz reaction is a coupling reaction in organic chemistry in which two alkyl halides are reacted with sodium metal in dry ether solution to form higher alkane. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in this reaction to obtain alkanes.
The general form of reaction equation is written as follows:
${\text{2R - X + 2Na}} \to {\text{R - R + 2N}}{{\text{a}}^{\text{ + }}}{{\text{X}}^{\text{ - }}}$
The above reaction defines that the two R groups are joined, yielding an alkane with a longer chain along with ${\text{NaX}}$, where X is halogen.
In the above question, we cannot form cyclohexane using this method. So, option D will be the least effective method for preparing cyclohexane. All other reactions are simple alkanes so they can be used for making cyclohexane.
So, the correct answer is Option D .
Note:
There are certain limitations of this reaction:
1.only symmetric alkanes can be synthesized via this method as a mixture of alkane products are formed when dissimilar alkanes are reacted.
2.methane cannot be obtained using this reaction because of the fact that an organic coupling reaction must have at least two carbon atoms.
3.This generally fails for the tertiary alkyl halides.
Reaction limitations must be remembered while forming the products for the given reactants. This reaction takes place in the presence of sodium metal and dry ether.
Complete step by step answer:
Wurtz reaction is a coupling reaction in organic chemistry in which two alkyl halides are reacted with sodium metal in dry ether solution to form higher alkane. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in this reaction to obtain alkanes.
The general form of reaction equation is written as follows:
${\text{2R - X + 2Na}} \to {\text{R - R + 2N}}{{\text{a}}^{\text{ + }}}{{\text{X}}^{\text{ - }}}$
The above reaction defines that the two R groups are joined, yielding an alkane with a longer chain along with ${\text{NaX}}$, where X is halogen.
In the above question, we cannot form cyclohexane using this method. So, option D will be the least effective method for preparing cyclohexane. All other reactions are simple alkanes so they can be used for making cyclohexane.
So, the correct answer is Option D .
Note:
There are certain limitations of this reaction:
1.only symmetric alkanes can be synthesized via this method as a mixture of alkane products are formed when dissimilar alkanes are reacted.
2.methane cannot be obtained using this reaction because of the fact that an organic coupling reaction must have at least two carbon atoms.
3.This generally fails for the tertiary alkyl halides.
Reaction limitations must be remembered while forming the products for the given reactants. This reaction takes place in the presence of sodium metal and dry ether.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




