
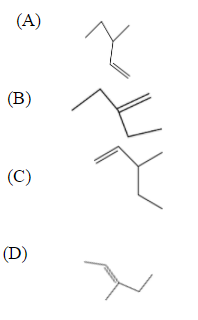
Which of the following is the most stable?

Answer
586.2k+ views
Hint: When chemical species have a tendency to release or donate electrons like the alkyl group in the carbon chain, the charge is relayed through the chain is positive (+I) Inductive effect.
Complete answer:
Alkenes having a more alkylated double bond is more stable due to the +I effect. Alkyl groups are known for donating electrons. Since double bonds contain $s{{p}^{2}}$ carbon atoms they are electronegative and require electrons. Alkyl groups show a positive inductive effect by releasing electrons. The stability of alkenes will be confirmed based on the measuring amount of energy associated with the hydrogenation of the molecule. The lower energy of molecules is more stable than higher energy molecules. More substituted alkenes are more stable than less substituted alkenes due to hyper conjugation. Three main things determine the stability of alkenes the number of substituents, their orientation, and hyper conjugation.
Option D compound has more conjugation than all other compounds, which is named as 2-ethyl-cis-pent-2-ene, which is a cis isomer. Based on the stability of alkenes on orientation cis isomer is more stable than trans and has more alkyl substituents. Trans isomer is less stable due to steric hindrance. The number of substituents in this compound. Hence it is more stable. Moreover, the stability of alkenes is based on the no of alkyl groups substituents.
So option D is the correct answer.
Note:
Alkenes have substituents, hydrogen atoms attached to the carbons in the double bonds. The more substituents the alkenes have, the more stable they are. E isomer in alkene means highly priority groups opposite side and Z isomer in alkene which is highly priority groups on the same side. Z isomer is more stable than E isomer because highly steric hindrance in E isomer leads to less stability.
Complete answer:
Alkenes having a more alkylated double bond is more stable due to the +I effect. Alkyl groups are known for donating electrons. Since double bonds contain $s{{p}^{2}}$ carbon atoms they are electronegative and require electrons. Alkyl groups show a positive inductive effect by releasing electrons. The stability of alkenes will be confirmed based on the measuring amount of energy associated with the hydrogenation of the molecule. The lower energy of molecules is more stable than higher energy molecules. More substituted alkenes are more stable than less substituted alkenes due to hyper conjugation. Three main things determine the stability of alkenes the number of substituents, their orientation, and hyper conjugation.
Option D compound has more conjugation than all other compounds, which is named as 2-ethyl-cis-pent-2-ene, which is a cis isomer. Based on the stability of alkenes on orientation cis isomer is more stable than trans and has more alkyl substituents. Trans isomer is less stable due to steric hindrance. The number of substituents in this compound. Hence it is more stable. Moreover, the stability of alkenes is based on the no of alkyl groups substituents.
So option D is the correct answer.
Note:
Alkenes have substituents, hydrogen atoms attached to the carbons in the double bonds. The more substituents the alkenes have, the more stable they are. E isomer in alkene means highly priority groups opposite side and Z isomer in alkene which is highly priority groups on the same side. Z isomer is more stable than E isomer because highly steric hindrance in E isomer leads to less stability.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




