
Which of the following is tribasic acid?
A) \[{{\text{H}}_3}{\text{P}}{{\text{O}}_2}\]
B) \[{{\text{H}}_3}{\text{P}}{{\text{O}}_3}\]
C) \[{{\text{H}}_3}{\text{P}}{{\text{O}}_4}\]
D) \[{{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_7}\]
Answer
559.2k+ views
Hint: There are various definitions of acids and bases. As per the Arrhenius concept, acid is a proton donor in an aqueous solution while the base is the hydroxide ion donor in an aqueous solution. As per the Bronsted Lowry theory acid is a proton donor while a base is a proton acceptor.
According to the Lewis theory, acid is an electron-pair donor while the base is the electron acceptor.
Acids are also considered as electrophilic species that are deficient in electrons and the base is a nucleophile.
Complete step-by-step answer:
Now, here we have to determine the tribasic acid for which it is important to know the structures of the acids given.Here, all given are the acids of the phosphorus.
In Option(A) acid given is \[{{\text{H}}_3}{\text{P}}{{\text{O}}_2}\] its structure is as follows:
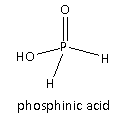
It is also called phosphinic acid. Here, only one ionizable hydrogen is present which is attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is one and it is monobasic acid. Therefore, option(A) is the incorrect answer to the question.
In Option(B) acid given is \[{{\text{H}}_3}{\text{P}}{{\text{O}}_3}\] its structure is as follows:
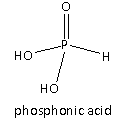
It is also called phosphonic acid. There are two ionizable hydrogens present which are attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is two hence, it is dibasic acid. Therefore, option(B) is also an incorrect answer to the question.
In Option(C) acid given is \[{{\text{H}}_3}{\text{P}}{{\text{O}}_4}\] its structure is as follows:
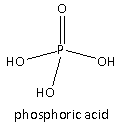
It is also called phosphoric acid. There are three ionizable hydrogens present which are attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is three hence, it is tribasic acid. Therefore, option(C) is the correct answer to the question.
In Option(D) acid given is \[{{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_7}\] its structure is as follows:
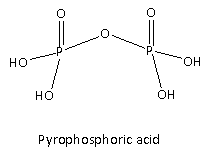
It is also called pyrophosphoric acid. There are four ionizable hydrogens present which are attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is four hence, it is tetrabasic acid. Therefore, option(D) is incorrect.
Note:
i) The basicity is given for acid while the acidity is given for the base.
ii) The basicity is the numbers of the ionizable hydrogen ions present in the acid and acidity is the numbers of replaceable hydroxide ions in the base.
iii) The prefix mono, di, tri, tetra, etc indicates the numbers of the replaceable hydroxide ions in the case of the acids. Here, in the question acid given is tribasic means there are three replaceable hydroxide ions are there in the acid.
According to the Lewis theory, acid is an electron-pair donor while the base is the electron acceptor.
Acids are also considered as electrophilic species that are deficient in electrons and the base is a nucleophile.
Complete step-by-step answer:
Now, here we have to determine the tribasic acid for which it is important to know the structures of the acids given.Here, all given are the acids of the phosphorus.
In Option(A) acid given is \[{{\text{H}}_3}{\text{P}}{{\text{O}}_2}\] its structure is as follows:

It is also called phosphinic acid. Here, only one ionizable hydrogen is present which is attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is one and it is monobasic acid. Therefore, option(A) is the incorrect answer to the question.
In Option(B) acid given is \[{{\text{H}}_3}{\text{P}}{{\text{O}}_3}\] its structure is as follows:

It is also called phosphonic acid. There are two ionizable hydrogens present which are attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is two hence, it is dibasic acid. Therefore, option(B) is also an incorrect answer to the question.
In Option(C) acid given is \[{{\text{H}}_3}{\text{P}}{{\text{O}}_4}\] its structure is as follows:

It is also called phosphoric acid. There are three ionizable hydrogens present which are attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is three hence, it is tribasic acid. Therefore, option(C) is the correct answer to the question.
In Option(D) acid given is \[{{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_7}\] its structure is as follows:

It is also called pyrophosphoric acid. There are four ionizable hydrogens present which are attached to the oxygen of the \[ - {\text{OH}}\] group hence, the basicity of the given acid is four hence, it is tetrabasic acid. Therefore, option(D) is incorrect.
Note:
i) The basicity is given for acid while the acidity is given for the base.
ii) The basicity is the numbers of the ionizable hydrogen ions present in the acid and acidity is the numbers of replaceable hydroxide ions in the base.
iii) The prefix mono, di, tri, tetra, etc indicates the numbers of the replaceable hydroxide ions in the case of the acids. Here, in the question acid given is tribasic means there are three replaceable hydroxide ions are there in the acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




