
Which of the following is Z isomer?
A.

B.
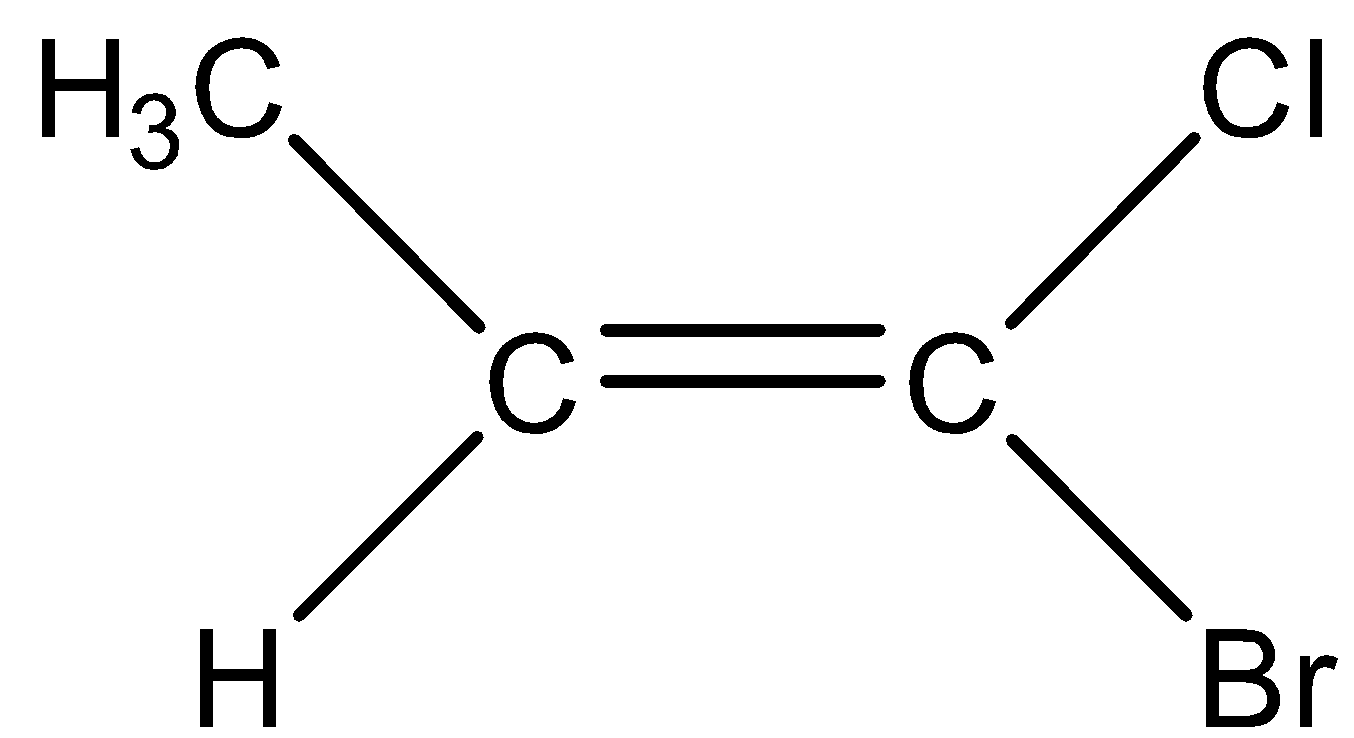
C.
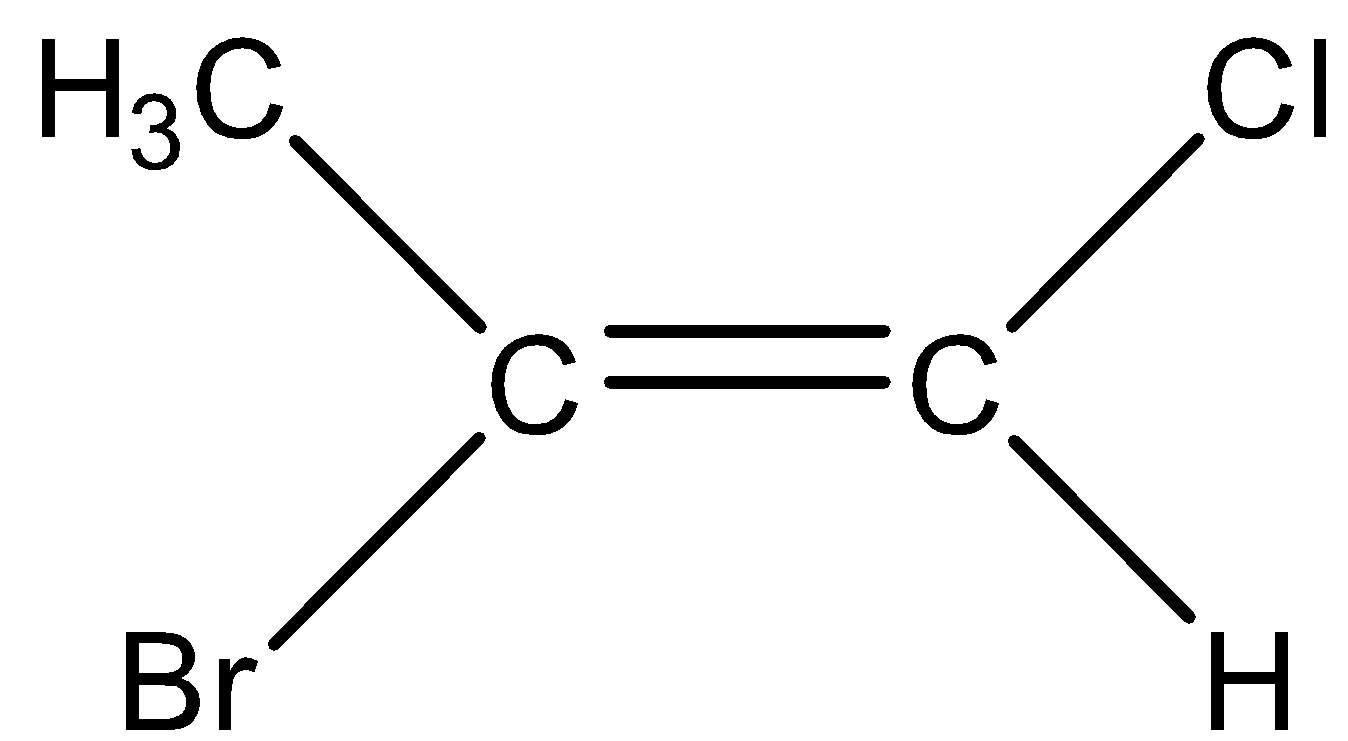
D.
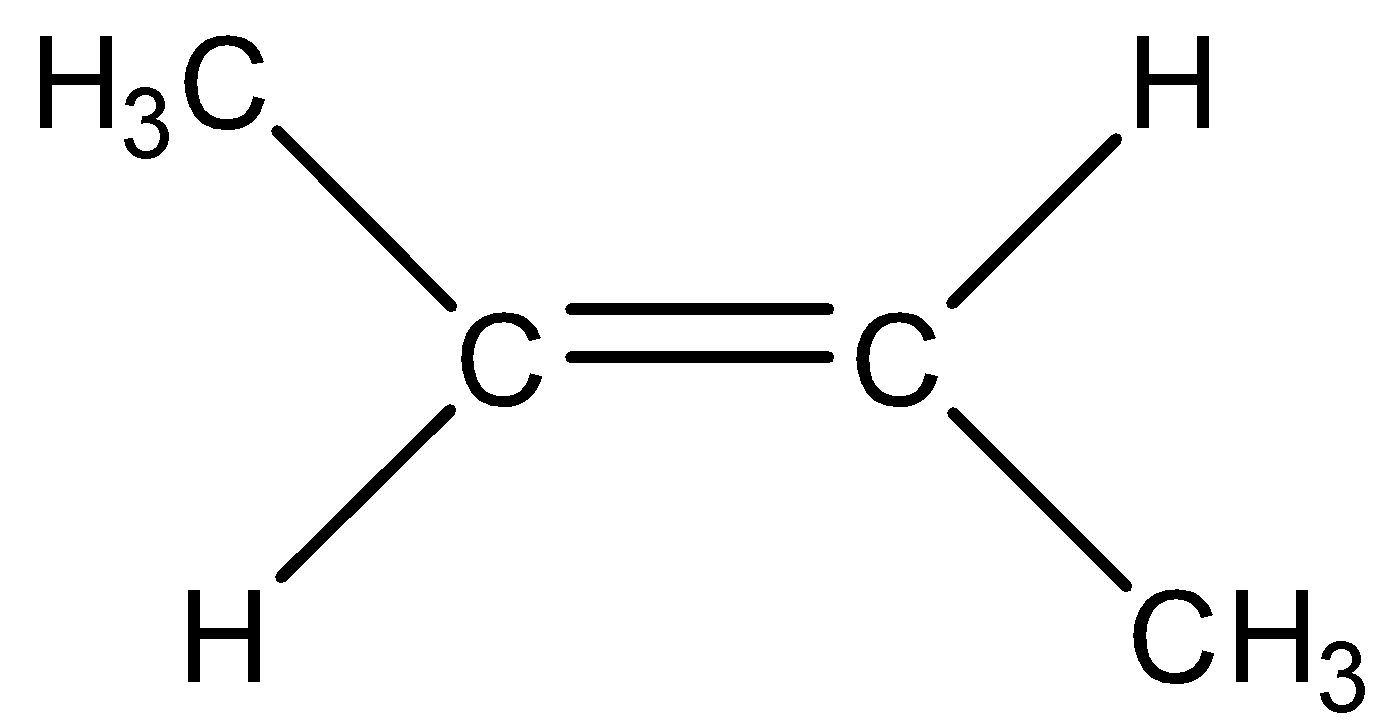




Answer
571.5k+ views
Hint: As we know that in alkenes there are two different substituents present at the each end of the carbon-carbon double bond that can occur as a pair of stereoisomers. The designators E and Z are very helpful to unambiguously designate the stereochemistry of alkene than the trans and cis.
Complete step by step solution:
In order to use the naming system, first we have to identify the higher priority group which is located at the each carbon of the double bond by using the similar priority rules we use in the case of R/S systems. When there is the presence of a higher priority group on the same side of the double bond then we designate it as Z-alkene. When there is the presence of the higher priority group on the opposite side of the double bond then we designate it as E-alkene.
The priority of the compounds is given based on their atomic number. In the left hand side, $C{H_3}$ has the highest priority when compared to hydrogen as the atomic number of carbon is greater when compared to hydrogen. On the right hand, the bromine has the highest priority when compared to chlorine as the atomic number of chlorine is less when compared to Bromine.
So, Option (A) is correct.
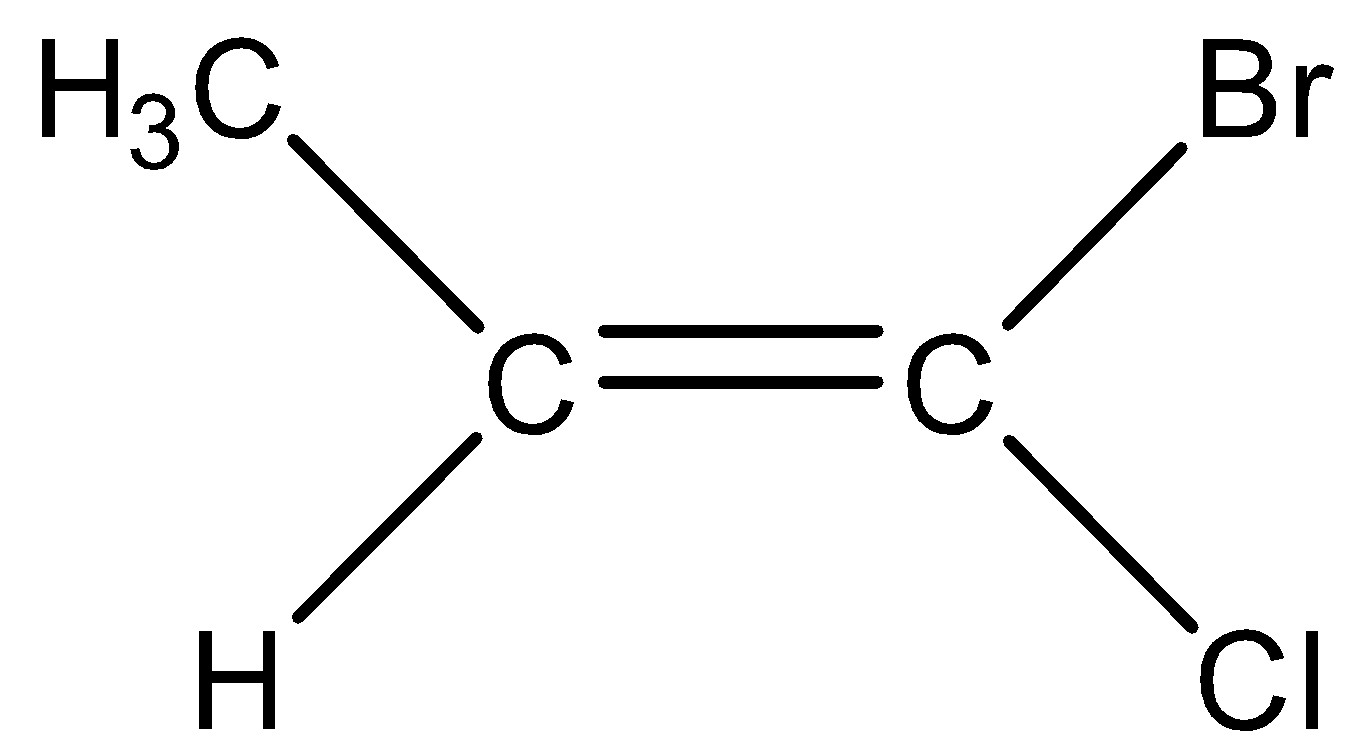
Note:We have to know that there is no possibility of designating all the alkenes as E or Z. When one of the double bonded carbon contains the same substituents, the alkene isn't considered as stereogenic and in such cases there is no possibility to designate E or Z configuration.
Complete step by step solution:
In order to use the naming system, first we have to identify the higher priority group which is located at the each carbon of the double bond by using the similar priority rules we use in the case of R/S systems. When there is the presence of a higher priority group on the same side of the double bond then we designate it as Z-alkene. When there is the presence of the higher priority group on the opposite side of the double bond then we designate it as E-alkene.
The priority of the compounds is given based on their atomic number. In the left hand side, $C{H_3}$ has the highest priority when compared to hydrogen as the atomic number of carbon is greater when compared to hydrogen. On the right hand, the bromine has the highest priority when compared to chlorine as the atomic number of chlorine is less when compared to Bromine.
So, Option (A) is correct.

Note:We have to know that there is no possibility of designating all the alkenes as E or Z. When one of the double bonded carbon contains the same substituents, the alkene isn't considered as stereogenic and in such cases there is no possibility to designate E or Z configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




