
Which of the following is/are correctly matched?
a.) \[[Ni{{(CO)}_{4}}]-ds{{p}^{2}}\] and diamagnetic
b.) \[[Ni{{(en)}_{3}}]{{(N{{O}_{2}})}_{2}}-s{{p}^{3}}{{d}^{2}}\] and two unpaired electrons
c.) \[[V{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}-s{{p}^{3}}{{d}^{2}}\] and two unpaired electrons
d.) \[[Mn{{(N{{O}^{+}})}_{3}}(CO)]-s{{p}^{3}}\]and diamagnetic
Answer
596.1k+ views
Hint: In order to solve this question, check the oxidation state of the central atom of each compound. Using that, predict the compound’s hybridization. If any orbital contains unpaired electrons, it is paramagnetic, otherwise it is diamagnetic.
Complete step by step solution:
Let us look at all the compounds one by one.
\[[Ni{{(CO)}_{4}}]\]
Oxidation state of Ni = 0.
Its electronic configuration is - \[[Ar]3{{d}^{8}}4{{s}^{2}}\]
Carbon monoxide is a strong field ligand. Therefore, pairing of electrons takes place. This can be represented as –
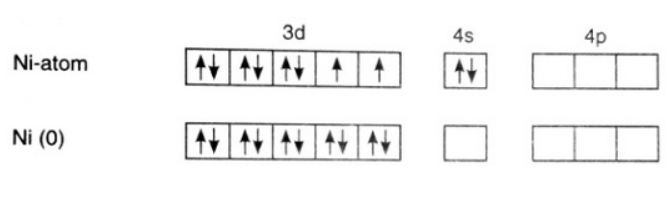
The hybridization of the compound is – \[s{{p}^{3}}\].
As all electrons in this compound are paired, it is a diamagnetic compound.
Option (a) is incorrect.
\[[Ni{{(en)}_{3}}]{{(N{{O}_{2}})}_{2}}\]
Oxidation state of Ni = +2
Its electronic configuration is - \[[Ar]3{{d}^{8}}4{{s}^{0}}\]
Ethylene diamine is a weak field ligand. Hence, there is no pairing of electrons. This can be represented as –
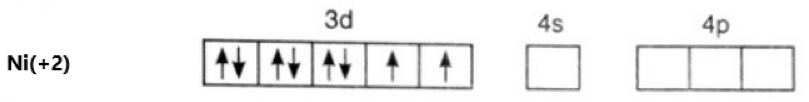
The hybridization of the compound is – \[s{{p}^{3}}{{d}^{2}}\].
As we can see, 2 electrons in 3d are unpaired, it is a paramagnetic compound.
Option (b) is correct.
\[[V{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
Oxidation state of V = +3
Its electronic configuration is - \[[Ar]3{{d}^{2}}4{{s}^{0}}\]
Ammonia is a strong field ligand. Therefore, pairing of electrons takes place. This can be represented as –
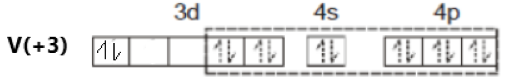
The hybridization of the compound is – \[{{d}^{2}}s{{p}^{3}}\].
As all electrons in this compound are paired, it is a diamagnetic compound.
Option (c) is incorrect.
\[[Mn{{(N{{O}^{+}})}_{3}}(CO)]\]
Oxidation state of Mn = +2
Its electronic configuration is - \[[Ar]3{{d}^{5}}4{{s}^{0}}\]
Nitrosonium is a weak field ligand. Hence, there is no pairing of electrons. This can be represented as –
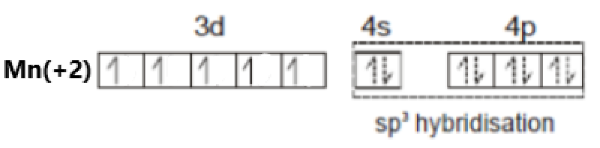
The hybridization of the compound is – \[s{{p}^{3}}\].
As all electrons in this compound are unpaired, it is a paramagnetic compound.
Option (d) is incorrect.
Therefore, the answer is – option (b).
Note: Compounds not attracted to magnetic fields are known as diamagnetic compounds. They have all paired electrons. Whereas, compounds which are attracted to magnetic fields, are known as paramagnetic compounds. They have unpaired electrons.
Complete step by step solution:
Let us look at all the compounds one by one.
\[[Ni{{(CO)}_{4}}]\]
Oxidation state of Ni = 0.
Its electronic configuration is - \[[Ar]3{{d}^{8}}4{{s}^{2}}\]
Carbon monoxide is a strong field ligand. Therefore, pairing of electrons takes place. This can be represented as –

The hybridization of the compound is – \[s{{p}^{3}}\].
As all electrons in this compound are paired, it is a diamagnetic compound.
Option (a) is incorrect.
\[[Ni{{(en)}_{3}}]{{(N{{O}_{2}})}_{2}}\]
Oxidation state of Ni = +2
Its electronic configuration is - \[[Ar]3{{d}^{8}}4{{s}^{0}}\]
Ethylene diamine is a weak field ligand. Hence, there is no pairing of electrons. This can be represented as –

The hybridization of the compound is – \[s{{p}^{3}}{{d}^{2}}\].
As we can see, 2 electrons in 3d are unpaired, it is a paramagnetic compound.
Option (b) is correct.
\[[V{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
Oxidation state of V = +3
Its electronic configuration is - \[[Ar]3{{d}^{2}}4{{s}^{0}}\]
Ammonia is a strong field ligand. Therefore, pairing of electrons takes place. This can be represented as –

The hybridization of the compound is – \[{{d}^{2}}s{{p}^{3}}\].
As all electrons in this compound are paired, it is a diamagnetic compound.
Option (c) is incorrect.
\[[Mn{{(N{{O}^{+}})}_{3}}(CO)]\]
Oxidation state of Mn = +2
Its electronic configuration is - \[[Ar]3{{d}^{5}}4{{s}^{0}}\]
Nitrosonium is a weak field ligand. Hence, there is no pairing of electrons. This can be represented as –

The hybridization of the compound is – \[s{{p}^{3}}\].
As all electrons in this compound are unpaired, it is a paramagnetic compound.
Option (d) is incorrect.
Therefore, the answer is – option (b).
Note: Compounds not attracted to magnetic fields are known as diamagnetic compounds. They have all paired electrons. Whereas, compounds which are attracted to magnetic fields, are known as paramagnetic compounds. They have unpaired electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




