
Which of the following is/are examples of unimolecular reaction?
(A) $ {{\text{O}}_{\text{3}}} \to {{\text{O}}_2} + {\text{O}} $
(B)
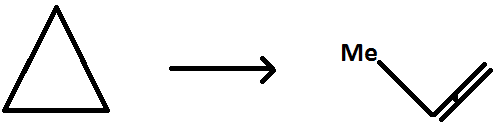
(C) $ {\text{NO + }}{{\text{O}}_{\text{3}}} \to {\text{N}}{{\text{O}}_2} + {{\text{O}}_2} $
(D) $ {\text{O + NO + }}{{\text{N}}_2} \to {\text{N}}{{\text{O}}_2} + {{\text{N}}_2} $

Answer
546.6k+ views
Hint: When a single molecule rearranges itself in a chemical reaction to form new products then this type of reaction is known as unimolecular reaction. Only one molecule participates in unimolecular reactions.
Complete step by step answer:
Reaction: The chemical process in which one or more chemicals change themselves by the rearrangement of atoms into new products.
Unimolecular reaction: When a single molecule rearranges itself to form one or more new products, these types of reactions are known as unimolecular reactions. Simply we can say that in unimolecular reactions a single reactant takes part in the reaction.
When two molecules rearrange themselves to form new products then this type of reaction is known as bimolecular and similarly trimolecular reaction (three reactants taking part in reaction) exists in chemistry.
Some of the examples of unimolecular reactions are as follows:
Radioactive decay (a single particle disintegrates itself multiple times), thermal decomposition (in the presence of heat a single molecule dissociates into two or more new products), racemisation, etc.
As we can see that option (A) has only one reactant i.e. ozone ( $ {{\text{O}}_{\text{3}}} $ ). So this is a unimolecular reaction.
Similarly option (B) also has one reactant therefore it is a unimolecular reaction.
Option (C) is a bimolecular reaction and option (D) is a trimolecular reaction.
Hence option (A) and option (B) are the correct answer.
Note:
The number of molecules that come together in a reaction as reactant is known as the molecularity of the reaction. The molecularity of the reaction is calculated as the sum of the coefficients of reactant species.
Complete step by step answer:
Reaction: The chemical process in which one or more chemicals change themselves by the rearrangement of atoms into new products.
Unimolecular reaction: When a single molecule rearranges itself to form one or more new products, these types of reactions are known as unimolecular reactions. Simply we can say that in unimolecular reactions a single reactant takes part in the reaction.
When two molecules rearrange themselves to form new products then this type of reaction is known as bimolecular and similarly trimolecular reaction (three reactants taking part in reaction) exists in chemistry.
Some of the examples of unimolecular reactions are as follows:
Radioactive decay (a single particle disintegrates itself multiple times), thermal decomposition (in the presence of heat a single molecule dissociates into two or more new products), racemisation, etc.
As we can see that option (A) has only one reactant i.e. ozone ( $ {{\text{O}}_{\text{3}}} $ ). So this is a unimolecular reaction.
Similarly option (B) also has one reactant therefore it is a unimolecular reaction.
Option (C) is a bimolecular reaction and option (D) is a trimolecular reaction.
Hence option (A) and option (B) are the correct answer.
Note:
The number of molecules that come together in a reaction as reactant is known as the molecularity of the reaction. The molecularity of the reaction is calculated as the sum of the coefficients of reactant species.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




