
Which of the following molecules has $3C-4{{e}^{-}}$ bond?
A. $A{{l}_{2}}C{{l}_{6}}$
B. $B{{e}_{2}}C{{l}_{4}}$
C. ${{I}_{2}}C{{l}_{6}}$
D. All of these
Answer
566.7k+ views
Hint: The 3-center 4-electron bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur tetrafluoride, the xenon fluorides, and the bifluoride ion. In the given compounds chlorine atoms will act as bridges and form one covalent bond and other coordinate bond.
Complete step by step answer:
Structure of $A{{l}_{2}}C{{l}_{6}}$: As we know aluminum atom has 3 electrons in its valence shell therefore it is trivalent but this trivalency makes its molecules electron deficient molecules where Al atom has one empty orbital. Then when it gets combined with the atom like chlorine that has 3 lone pairs, back bonding happens where chlorine donates its lone pair in the vacant orbital of Al and fulfills its octet. That’s how the dimerization of aluminum trichloride takes place.
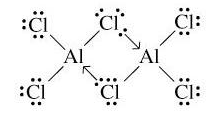
Now the important point is why is it called a $3C-4{{e}^{-}}$ bond?
To understand that, observe the banana bonds. If you start from one aluminum atom and via chlorine ends up on another aluminum atom, you will find a banana shape, that’s why it is called a banana bond. Now in one bond there are three atoms involved, two are aluminum and one is chlorine therefore 3 centered. Also each banana bond involves 4 electrons, two from covalent bond and two from co-ordinate bond. Therefore, 4 electrons.
That’s why it is called a $3C-4{{e}^{-}}$ bond.
Structure of $B{{e}_{2}}C{{l}_{4}}$: Similarly beryllium also has vacant orbitals therefore chlorine donate its lone pair to that vacant orbital and dimerisation of $BeC{{l}_{2}}$takes place.
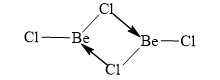
So it also has a $3C-4{{e}^{-}}$ bond.
Structure of ${{I}_{2}}C{{l}_{6}}$: Its structure is very much similar to $A{{l}_{2}}C{{l}_{6}}$ where you can simply replace All by iodine. So it also has a $3C-4{{e}^{-}}$bond.
Hence the answer is option B.
Additional information:
Aluminum chloride is a powerful Lewis acid, capable of forming stable Lewis acid-base compounds with even Lewis bases.
Note: To have back bonding it is important to have a vacant orbital for acceptor atom and a lone pair for the donor atom. Also both the elements must belong to either the 2nd period or 3rd period. Because of back bonding formation of π-bond takes place which decreases the bond length.
Complete step by step answer:
Structure of $A{{l}_{2}}C{{l}_{6}}$: As we know aluminum atom has 3 electrons in its valence shell therefore it is trivalent but this trivalency makes its molecules electron deficient molecules where Al atom has one empty orbital. Then when it gets combined with the atom like chlorine that has 3 lone pairs, back bonding happens where chlorine donates its lone pair in the vacant orbital of Al and fulfills its octet. That’s how the dimerization of aluminum trichloride takes place.

Now the important point is why is it called a $3C-4{{e}^{-}}$ bond?
To understand that, observe the banana bonds. If you start from one aluminum atom and via chlorine ends up on another aluminum atom, you will find a banana shape, that’s why it is called a banana bond. Now in one bond there are three atoms involved, two are aluminum and one is chlorine therefore 3 centered. Also each banana bond involves 4 electrons, two from covalent bond and two from co-ordinate bond. Therefore, 4 electrons.
That’s why it is called a $3C-4{{e}^{-}}$ bond.
Structure of $B{{e}_{2}}C{{l}_{4}}$: Similarly beryllium also has vacant orbitals therefore chlorine donate its lone pair to that vacant orbital and dimerisation of $BeC{{l}_{2}}$takes place.

So it also has a $3C-4{{e}^{-}}$ bond.
Structure of ${{I}_{2}}C{{l}_{6}}$: Its structure is very much similar to $A{{l}_{2}}C{{l}_{6}}$ where you can simply replace All by iodine. So it also has a $3C-4{{e}^{-}}$bond.
Hence the answer is option B.
Additional information:
Aluminum chloride is a powerful Lewis acid, capable of forming stable Lewis acid-base compounds with even Lewis bases.
Note: To have back bonding it is important to have a vacant orbital for acceptor atom and a lone pair for the donor atom. Also both the elements must belong to either the 2nd period or 3rd period. Because of back bonding formation of π-bond takes place which decreases the bond length.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




