
Which of the following nitroalkane is a tertiary nitroalkane?
(a) 2-methyl-1-nitropropane
(b) 2-nitrobutane
(c) 1-nitrobutane
(d) 2-methyl-2-nitropropane
Answer
583.2k+ views
Hint: Nitroalkane contains the nitro group and the alkane and tertiary nitroalkane is that in which the carbon atom bonded to the nitro group contains the three-alkyl group. Now identify it.
Complete step by step answer:
Alkanes are the simplest hydrocarbon in which the carbon atoms are connected to each other through the C-C single bond and there is no double in between them, thus, also called as the saturated hydrocarbon. Their general chemical formula is ${{C}_{n}}{{H}_{2n}}$, here n is the number of the carbon atoms present in the molecule. Examples are methane (consisting of one carbon atom), ethane( consisting of two carbon atoms) ,propane (consisting of three carbon atoms) and so on.
When a nitro group is attached to the alkanes, then the alkanes are known as the nitroalkanes.
Depending on the number on the alkyl group (R-C) , attached to the carbon, the nitroalkanes can be classified into the following three categories:
1. Primary nitroalkanes: In this, one alkyl group is attached to the carbon atom bonded to the -OH group.
2. Secondary nitroalkanes: In this, two alkyl groups are attached to the carbon atom bonded to the -OH group.
3. Tertiary nitroalkanes: In this, three alkyl groups are attached to the carbon atom bonded to the -OH group.
So, to know which of the following from the above is the tertiary nitroalkane we will first have to write their structures.
So, from the above drawn structures of all nitroalkanes given, we can see that 2-methyl-2-nitropropane is the tertiary nitroalkane as its carbon atom is bonded to the three alkyl groups.
Hence, option(d) is correct.
Note: The aliphatic nitroalkanes are generally colorless while the aromatic nitroalkanes are mostly colored and they are soluble in the water and have ,thus, high boiling points due to the strong interactions of it with the water.
Complete step by step answer:
Alkanes are the simplest hydrocarbon in which the carbon atoms are connected to each other through the C-C single bond and there is no double in between them, thus, also called as the saturated hydrocarbon. Their general chemical formula is ${{C}_{n}}{{H}_{2n}}$, here n is the number of the carbon atoms present in the molecule. Examples are methane (consisting of one carbon atom), ethane( consisting of two carbon atoms) ,propane (consisting of three carbon atoms) and so on.
When a nitro group is attached to the alkanes, then the alkanes are known as the nitroalkanes.
Depending on the number on the alkyl group (R-C) , attached to the carbon, the nitroalkanes can be classified into the following three categories:
1. Primary nitroalkanes: In this, one alkyl group is attached to the carbon atom bonded to the -OH group.
2. Secondary nitroalkanes: In this, two alkyl groups are attached to the carbon atom bonded to the -OH group.
3. Tertiary nitroalkanes: In this, three alkyl groups are attached to the carbon atom bonded to the -OH group.
So, to know which of the following from the above is the tertiary nitroalkane we will first have to write their structures.
| Sr. no | Nitroalkane | Structure |
| 1. | 2-methyl-1-nitropropane | 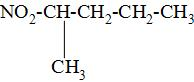
|
| 2 | 2-nitrobutane | 
|
| 3. | 1-nitrobutane | 
|
| 4. | 2-methyl-2-nitropropane | 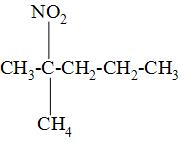
|
So, from the above drawn structures of all nitroalkanes given, we can see that 2-methyl-2-nitropropane is the tertiary nitroalkane as its carbon atom is bonded to the three alkyl groups.
Hence, option(d) is correct.
Note: The aliphatic nitroalkanes are generally colorless while the aromatic nitroalkanes are mostly colored and they are soluble in the water and have ,thus, high boiling points due to the strong interactions of it with the water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




