
Which of the following orbital electrons will be closer to the nucleus?
(A) 6s
(B) 4f
(C) 5d
(D) 6p
Answer
573.6k+ views
Hint: Orbital is the path in which the electrons move around the nucleus in the atom. We can say that the orbital which has the lowest value of principal quantum number, will be closest to the nucleus.
Complete answer:
We need to give the orbital which is closest to the nucleus from the given options. So, we need to find an orbital which has the lowest radius amongst the given.
- We know that an orbital is the path in which the electrons move around the nucleus in the atom.
- There are many orbitals present in the atoms. Depending upon their energy, they are filled by the electrons.
- In the designation of an orbital, the number shows the principal quantum number of the orbital. So, it shows the shell or orbit of the orbital. The shells are named as K, L, M, N,… They are designed with the numbers 1, 2, 3,…
- We can say that lower the value of the principal quantum number, closer the orbital will be to the nucleus.
- The alphabet after the principal quantum number shows the orbital. The orbital s, p, d…. shows azimuthal quantum number 1, 2, 3…
- Out of the given orbitals, the orbital 4f has the lowest value of principal quantum number. So, it will be the closest to the nucleus.
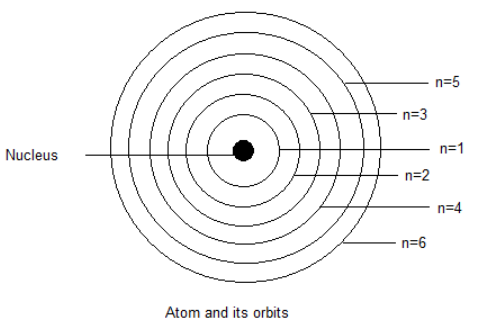
We can see in the picture that the orbits of the atoms are arranged in certain order and we can observe their distance from the nucleus. We can say that lower the principal quantum number, less far it will be from the nucleus.
So, the correct answer to this question is (B).
Note:
Note that 6s orbital is filled before 4f orbital because 6s orbital has less energy than 4f orbital. But that does not mean that 6s orbital is closer to the nucleus than 4f orbital. Thus, we can say that we just need to compare the principal quantum number in order to find which orbital is closer.
Complete answer:
We need to give the orbital which is closest to the nucleus from the given options. So, we need to find an orbital which has the lowest radius amongst the given.
- We know that an orbital is the path in which the electrons move around the nucleus in the atom.
- There are many orbitals present in the atoms. Depending upon their energy, they are filled by the electrons.
- In the designation of an orbital, the number shows the principal quantum number of the orbital. So, it shows the shell or orbit of the orbital. The shells are named as K, L, M, N,… They are designed with the numbers 1, 2, 3,…
- We can say that lower the value of the principal quantum number, closer the orbital will be to the nucleus.
- The alphabet after the principal quantum number shows the orbital. The orbital s, p, d…. shows azimuthal quantum number 1, 2, 3…
- Out of the given orbitals, the orbital 4f has the lowest value of principal quantum number. So, it will be the closest to the nucleus.

We can see in the picture that the orbits of the atoms are arranged in certain order and we can observe their distance from the nucleus. We can say that lower the principal quantum number, less far it will be from the nucleus.
So, the correct answer to this question is (B).
Note:
Note that 6s orbital is filled before 4f orbital because 6s orbital has less energy than 4f orbital. But that does not mean that 6s orbital is closer to the nucleus than 4f orbital. Thus, we can say that we just need to compare the principal quantum number in order to find which orbital is closer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




