
Which of the following oxo-acids of sulphur has more than one oxidation state of oxygen?
A. ${H_2}{S_2}{O_3}$
B. ${H_2}{S_2}{O_4}$
C. ${H_2}{S_2}{O_6}$
D. ${H_2}{S_2}{O_8}$
Answer
585.6k+ views
Hint: An oxo acid contains oxygen as the name suggests oxo acids of sulphur are the acids containing oxygen and sulphur. Oxidation state is the number of electrons lost during chemical bond formation.
Complete step by step solution: The oxoacids of sulphur are more numerous as sulphur can share six electrons. Many of the oxoacids of sulphur do not exist as free acids but are called anions and salts.

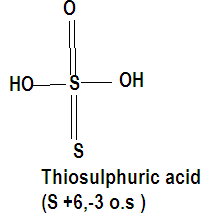
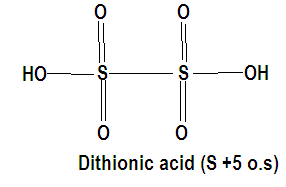

On analyzing the given compounds and making their structures we observe that sulphur has more than one oxidation state in thiosulphuric acid which is +6 and -3 where the central sulphur atom has a +6 oxidation state as it is sharing all six of its electrons. Similarly we have calculated the oxidation state of sulphur in every compound where all the oxygen atoms have made either a double bond or a O-H bond with sulphur.
The oxygen in peroxodisulfuric acid has O-O linkage This linkage is called peroxy linkage. Here the oxygen atom forms a single bond with the other oxygen atom and each of them has a -1 charge this linkage is a weak linkage but peroxodisulfuric acid is the compound with more than one oxidation states of oxygen as the oxygen atoms in this compound has the oxidation state (-1 and +6).therefore the correct option is option D.
Additional information: oxoanions have strong double bonds therefore they have little tendency to polymerize. Its acid series goes like:
- Sulphurous acid
- Sulphuric acid
- Thionic acid
- Peroxoacid
Note: Acids ending in -ous have S in the oxidation state +4 and form salts ending in –ite. Acids ending in –ic have S in the oxidation state +4 and form salts ending in –ate.
Complete step by step solution: The oxoacids of sulphur are more numerous as sulphur can share six electrons. Many of the oxoacids of sulphur do not exist as free acids but are called anions and salts.




On analyzing the given compounds and making their structures we observe that sulphur has more than one oxidation state in thiosulphuric acid which is +6 and -3 where the central sulphur atom has a +6 oxidation state as it is sharing all six of its electrons. Similarly we have calculated the oxidation state of sulphur in every compound where all the oxygen atoms have made either a double bond or a O-H bond with sulphur.
The oxygen in peroxodisulfuric acid has O-O linkage This linkage is called peroxy linkage. Here the oxygen atom forms a single bond with the other oxygen atom and each of them has a -1 charge this linkage is a weak linkage but peroxodisulfuric acid is the compound with more than one oxidation states of oxygen as the oxygen atoms in this compound has the oxidation state (-1 and +6).therefore the correct option is option D.
Additional information: oxoanions have strong double bonds therefore they have little tendency to polymerize. Its acid series goes like:
- Sulphurous acid
- Sulphuric acid
- Thionic acid
- Peroxoacid
Note: Acids ending in -ous have S in the oxidation state +4 and form salts ending in –ite. Acids ending in –ic have S in the oxidation state +4 and form salts ending in –ate.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




