
Which of the following oxyacids does not contain P-O-P bond?
A. Isohypophosphoric acid
B. Pyrophosphorous acid
C. Diphosphoric acid
D. Hypophosphoric acid.
Answer
516.6k+ views
Hint: The acid which contains oxygen is called oxyacid. If the oxygen is present in between two phosphorus atoms then the bond is called P-O-P bond. To find the P-O-P bond present in the given molecule the structure of the compound must be known.
Complete answer:
- In the question it is asked to find the compound which does not contain P-O-P bond in its structure among the given options.
- First of all, we should know the structure of the compounds which are present in the given options.
- Coming to option A, Isohypophosphoric acid.
- The structure of Isohypophosphoric acid is as follows.
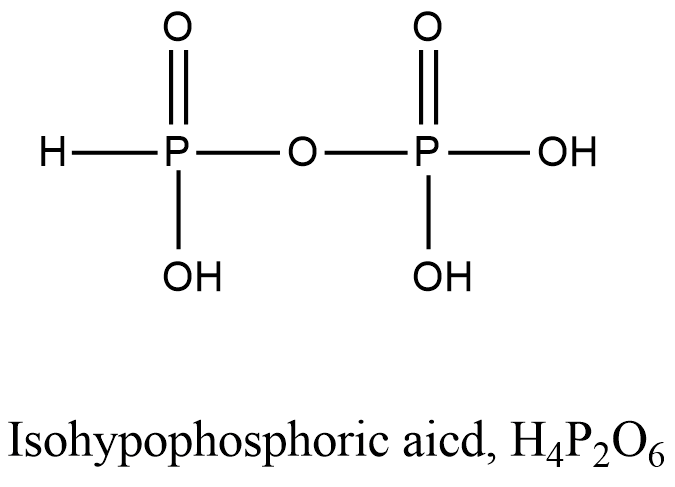
-In the structure of the Isohypophosphoric acid there is a presence of P-O-P bond.
- So, option A is incorrect.
- Coming to option B, Pyrophosphorous acid.
- The structure of Pyrophosphorous acid is as follows.
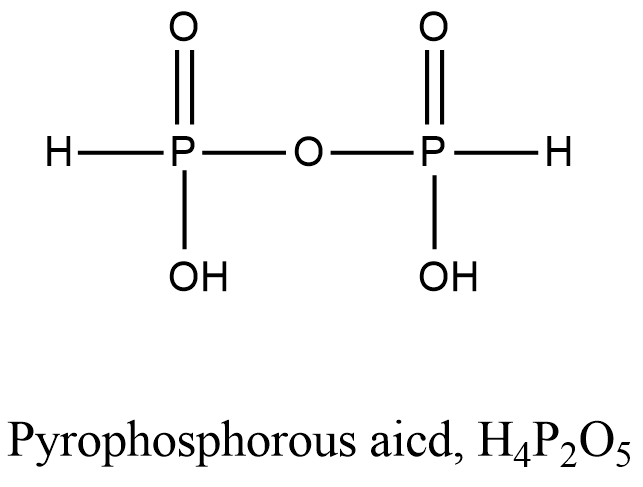
- In the structure of the Pyrophosphorous acid there is a presence of P-O-P bond.
- So, option B is incorrect.
- Coming to option C, Diphosphoric acid.
- The structure of Diphosphoric acid is as follows.
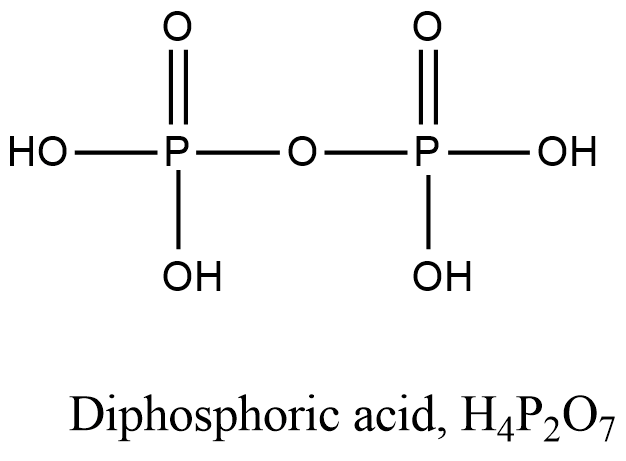
- In the structure of the Diphosphoric acid there is a presence of P-O-P bond.
- So, option C is incorrect.
- Coming to option D, Hypophosphoric acid.
- The structure of Hypophosphoric acid is as follows.
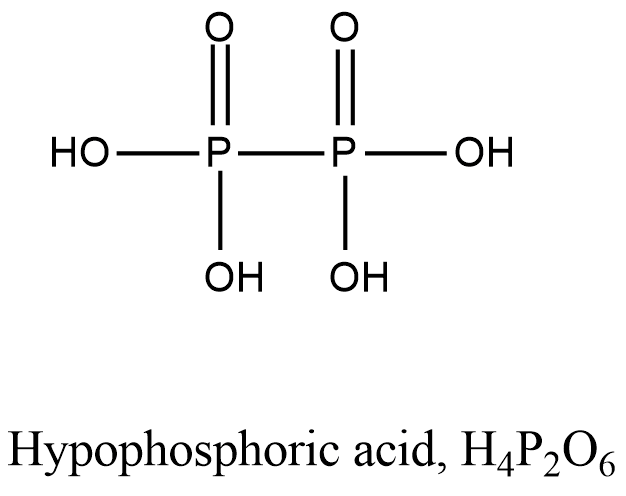
- In the above structure of the Hypophosphoric acid there is no P-O-P bond.
So, the option D is correct.
Note:
We have to draw the structures of the given compounds to know about the presence of the P-O-P bond. Without drawing the structures of the given compounds, we can find the presence of the P-O-P bond in the respective oxyacid.
Complete answer:
- In the question it is asked to find the compound which does not contain P-O-P bond in its structure among the given options.
- First of all, we should know the structure of the compounds which are present in the given options.
- Coming to option A, Isohypophosphoric acid.
- The structure of Isohypophosphoric acid is as follows.
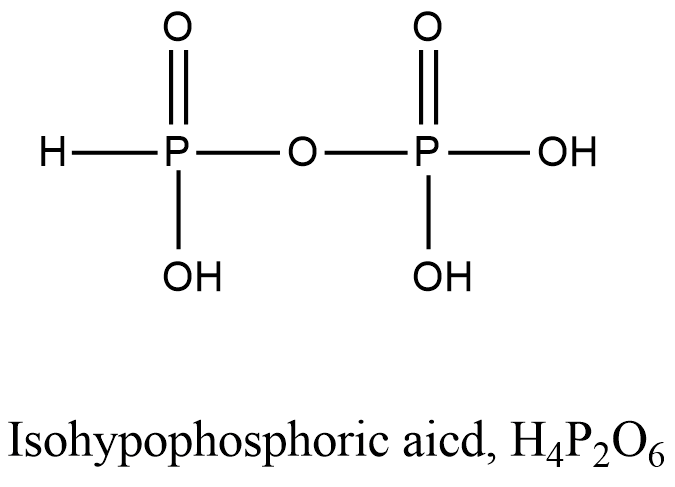
-In the structure of the Isohypophosphoric acid there is a presence of P-O-P bond.
- So, option A is incorrect.
- Coming to option B, Pyrophosphorous acid.
- The structure of Pyrophosphorous acid is as follows.

- In the structure of the Pyrophosphorous acid there is a presence of P-O-P bond.
- So, option B is incorrect.
- Coming to option C, Diphosphoric acid.
- The structure of Diphosphoric acid is as follows.

- In the structure of the Diphosphoric acid there is a presence of P-O-P bond.
- So, option C is incorrect.
- Coming to option D, Hypophosphoric acid.
- The structure of Hypophosphoric acid is as follows.

- In the above structure of the Hypophosphoric acid there is no P-O-P bond.
So, the option D is correct.
Note:
We have to draw the structures of the given compounds to know about the presence of the P-O-P bond. Without drawing the structures of the given compounds, we can find the presence of the P-O-P bond in the respective oxyacid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




