
Which of the following polymers has ester linkage?
A. Nylon
B. Bakelite
C. Terylene
D. PVC
Answer
578.1k+ views
Hint: An ester is a compound that is formed when a carboxylic acid reacts with an alcohol to yield an ester and releases a water molecule. This is a dehydration reaction. The bond that is formed after one proton and one hydroxyl group loses from the carboxylic acid and the alcohol respectively, is known as the ester linkage.
Complete answer:
Polyesters contain the ester linkage between monomer units. This type of bond is usually, but not exclusively, formed via either a transesterification reaction or a ring opening reaction. Terylene is a synthetic polyester fibre produced by polymerizing ethylene glycol and terephthalic acid which is obtained from petroleum. The structure of terylene is as follows:
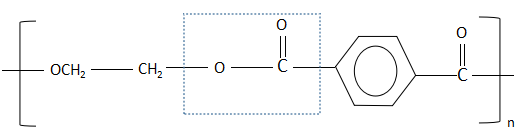
In the diagram, we can see that there are some bonds marked by blue dotted lines. These blue dotted lines are the ester linkage that has been formed between the ethylene glycol and the terephthalic acid.
In the case of nylon, it is a thermoplastic silky material that can be melt-processed into fibers, films, or shapes. It is made up of repeating units linked by amide links which is similar to the peptide bonds found in proteins.
In the case of Bakelite, it is a thermosetting phenol formaldehyde resin, which is formed from a condensation reaction of phenol with formaldehyde.
PVC is a synthetic plastic polymer. The electrolysis of salt water produces chlorine. The chlorine is then combined with ethylene that has been obtained from oil. The resulting element is ethylene dichloride, which is converted at very high temperatures to vinyl chloride monomer. These monomer molecules are polymerized forming polyvinyl chloride resin.
Thus, Terylene is the polymer that has an ester linkage in it.
So, the correct answer is Option C.
Note:
Terylene is an extensively used polymer in the textile industry. It is used to make hard wear clothes like sarees and other dress material. Terylene is made by a process of polymerizing ethylene glycol and terephthalic acid. It is also mixed with natural fibre like cotton and wool to make more variety of synthetic clothes.
Complete answer:
Polyesters contain the ester linkage between monomer units. This type of bond is usually, but not exclusively, formed via either a transesterification reaction or a ring opening reaction. Terylene is a synthetic polyester fibre produced by polymerizing ethylene glycol and terephthalic acid which is obtained from petroleum. The structure of terylene is as follows:
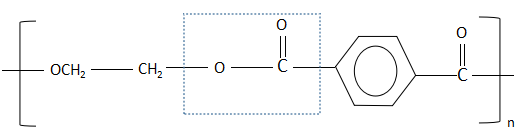
In the diagram, we can see that there are some bonds marked by blue dotted lines. These blue dotted lines are the ester linkage that has been formed between the ethylene glycol and the terephthalic acid.
In the case of nylon, it is a thermoplastic silky material that can be melt-processed into fibers, films, or shapes. It is made up of repeating units linked by amide links which is similar to the peptide bonds found in proteins.
In the case of Bakelite, it is a thermosetting phenol formaldehyde resin, which is formed from a condensation reaction of phenol with formaldehyde.
PVC is a synthetic plastic polymer. The electrolysis of salt water produces chlorine. The chlorine is then combined with ethylene that has been obtained from oil. The resulting element is ethylene dichloride, which is converted at very high temperatures to vinyl chloride monomer. These monomer molecules are polymerized forming polyvinyl chloride resin.
Thus, Terylene is the polymer that has an ester linkage in it.
So, the correct answer is Option C.
Note:
Terylene is an extensively used polymer in the textile industry. It is used to make hard wear clothes like sarees and other dress material. Terylene is made by a process of polymerizing ethylene glycol and terephthalic acid. It is also mixed with natural fibre like cotton and wool to make more variety of synthetic clothes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




