
Which of the following produce Oxonium salt with mineral acid?
(A) Diethyl ether
(B) Ethene
(C) Ethanoic acid
(D) None of the above
Answer
515.4k+ views
Hint: To proceed with this question, first of all we need to understand the concept of oxonium salts i.e the oxonium ions. So we will understand the formation of oxonium ions. Oxonium ions can be formed by the alcohols and the ethers in acidic conditions i.e. in the presence of mineral acids.
Complete answer:
As we know , oxonium ions can be formed from ether and the alcohols easily in acidic conditions.
So, hydrocarbon’s oxonium ions can be formed by the protonation or the alkylation of alcohols or the ethers, $ {R_1} - O - {R_2} $ . Where, R1 and R2 are two alkyl groups attached to the oxygen atom.
Now looking at our given options, let’s start with option A i.e. Diethyl ether.
So, we will write a chemical equation of reaction between the Diethyl ether with the mineral acid, let's say Hydrochloric acid, $ HCl $ .
So, let’s understand our chemical reaction structurally:
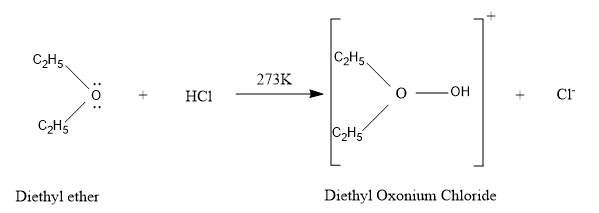
Hence, the reaction is very clear that Diethyl ether reacts with hydrochloric acid at the temperature of $ 273K $ and produces the Diethyl oxonium chloride and the chloride ions are released.
So, the correct answer is option A only. Therefore, Diethyl ether on reacting with mineral acid produces the oxonium ion i.e. the Diethyl oxonium chloride.
Note:
Here in the above reaction between hydrochloric acid and Diethyl ether i.e. in the production of oxonium ions, the ether bond changes to the hydroxyl bonds. Ether has changed into the alcohol ions with a positive charge on the whole molecule and the chloride ion is released in the above equation.
Complete answer:
As we know , oxonium ions can be formed from ether and the alcohols easily in acidic conditions.
So, hydrocarbon’s oxonium ions can be formed by the protonation or the alkylation of alcohols or the ethers, $ {R_1} - O - {R_2} $ . Where, R1 and R2 are two alkyl groups attached to the oxygen atom.
Now looking at our given options, let’s start with option A i.e. Diethyl ether.
So, we will write a chemical equation of reaction between the Diethyl ether with the mineral acid, let's say Hydrochloric acid, $ HCl $ .
So, let’s understand our chemical reaction structurally:

Hence, the reaction is very clear that Diethyl ether reacts with hydrochloric acid at the temperature of $ 273K $ and produces the Diethyl oxonium chloride and the chloride ions are released.
So, the correct answer is option A only. Therefore, Diethyl ether on reacting with mineral acid produces the oxonium ion i.e. the Diethyl oxonium chloride.
Note:
Here in the above reaction between hydrochloric acid and Diethyl ether i.e. in the production of oxonium ions, the ether bond changes to the hydroxyl bonds. Ether has changed into the alcohol ions with a positive charge on the whole molecule and the chloride ion is released in the above equation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




