
Which of the following produces chiral molecules upon treatment with Lindlar’s catalyst?
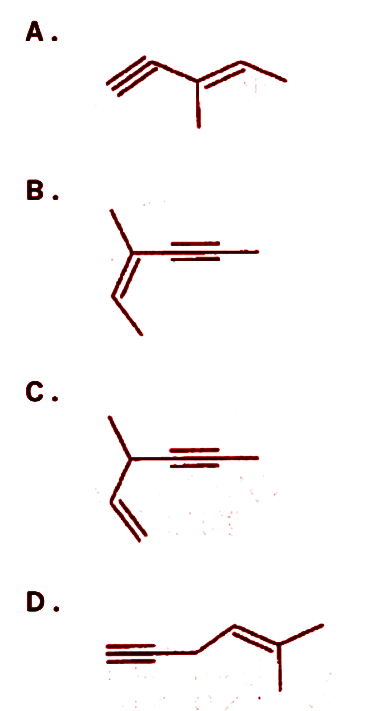

Answer
601.8k+ views
Hint: A chiral molecule will only be produced upon hydrogenation with Lindlar’s catalyst if one of the carbons involved in the alkyne bond is connected to a chiral carbon. With this information, observe each of the given structures very closely for a possible presence of the same.
Complete step-by-step answer:
Let us first understand what the Lindlar’s catalyst really is and its role in the hydrogenation of alkynes.
Lindlar’s catalyst is a “poisoned” metal catalyst that performs hydrogenations of alkynes in the presence of hydrogen gas (\[{{H}_{2}}\]). By “poisoned” we mean that it lacks the normal activity associated with palladium catalysts for reducing double bonds. This is useful because sometimes we’d like to start with an alkyne and go down one “rung” of the oxidation ladder to an alkene. But if you use normal palladium on carbon, you’ll get full reduction to the alkane.
Lindlar’s catalyst is a palladium catalyst poisoned with traces of lead and quinoline, that reduce its activity such that it can only reduce alkynes, not alkenes. It always gives the cis-alkene, in contrast to Na/NH3, which gives the trans alkenes.
A few examples of the partial oxidation of alkynes by Lindlar’s catalyst are:
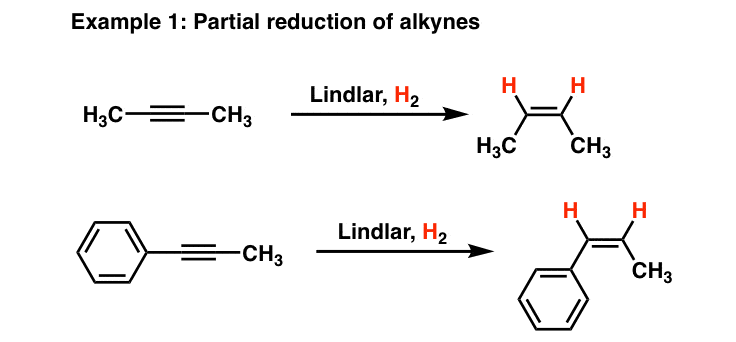
Now, let us try and understand what a chiral carbon atom really is.
An asymmetric carbon atom (chiral carbon) is a carbon atom that is attached to four different types of atoms or groups of atoms. This Carbon atom is essential for the existence of several stereoisomers as the number of stereoisomers of an organic compound is ${{2}^{n}}$, where n represents the number of asymmetric carbon atoms (unless there is an internal plane of symmetry.)
With this in mind, let us not try and find the presence of chiral carbon atoms in any of the four given alkynes.
We find that only in the alkyne in option c) does there exist a chiral Carbon atom, therefore that alkyne is the only one of the given options which does give out a chiral compound upon hydrogenation with the Lindlar’s catalyst. The reaction of which is as follows:

Note: Lindlar’s catalyst is commercially available but may also be prepared by the reduction of palladium chloride in a slurry of calcium carbonate followed by the addition of lead acetate. A variety of other "catalyst poisons" have been used, including lead oxide and quinoline. The palladium content of the supported catalyst is usually 5% by weight.
Complete step-by-step answer:
Let us first understand what the Lindlar’s catalyst really is and its role in the hydrogenation of alkynes.
Lindlar’s catalyst is a “poisoned” metal catalyst that performs hydrogenations of alkynes in the presence of hydrogen gas (\[{{H}_{2}}\]). By “poisoned” we mean that it lacks the normal activity associated with palladium catalysts for reducing double bonds. This is useful because sometimes we’d like to start with an alkyne and go down one “rung” of the oxidation ladder to an alkene. But if you use normal palladium on carbon, you’ll get full reduction to the alkane.
Lindlar’s catalyst is a palladium catalyst poisoned with traces of lead and quinoline, that reduce its activity such that it can only reduce alkynes, not alkenes. It always gives the cis-alkene, in contrast to Na/NH3, which gives the trans alkenes.
A few examples of the partial oxidation of alkynes by Lindlar’s catalyst are:

Now, let us try and understand what a chiral carbon atom really is.
An asymmetric carbon atom (chiral carbon) is a carbon atom that is attached to four different types of atoms or groups of atoms. This Carbon atom is essential for the existence of several stereoisomers as the number of stereoisomers of an organic compound is ${{2}^{n}}$, where n represents the number of asymmetric carbon atoms (unless there is an internal plane of symmetry.)
With this in mind, let us not try and find the presence of chiral carbon atoms in any of the four given alkynes.
We find that only in the alkyne in option c) does there exist a chiral Carbon atom, therefore that alkyne is the only one of the given options which does give out a chiral compound upon hydrogenation with the Lindlar’s catalyst. The reaction of which is as follows:

Note: Lindlar’s catalyst is commercially available but may also be prepared by the reduction of palladium chloride in a slurry of calcium carbonate followed by the addition of lead acetate. A variety of other "catalyst poisons" have been used, including lead oxide and quinoline. The palladium content of the supported catalyst is usually 5% by weight.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




