
Which of the following reactions are both stereospecific and stereoselective?
This question has multiple answers
A) SN1
B) SN2
C) E1
D) E2
Answer
510.9k+ views
Hint: We have to know that in stereoselective reactions two isomers are formed out of which one is major and other is minor. On the other hand, a stereospecific reaction is the one in which one stereoisomer is formed. In SN2 reactions transition state is formed whereas in SN1 reactions ,carbocation is formed as an intermediate.
Complete answer:
We will look at all the options to answer this question.
Option A) SN1 reactions are the one in which carbocation as an intermediate is formed and nucleophile can attack from both the positions, this reaction is unimolecular and rate depends only on the first step. So this reaction is non-stereospecific, thus this option is incorrect.
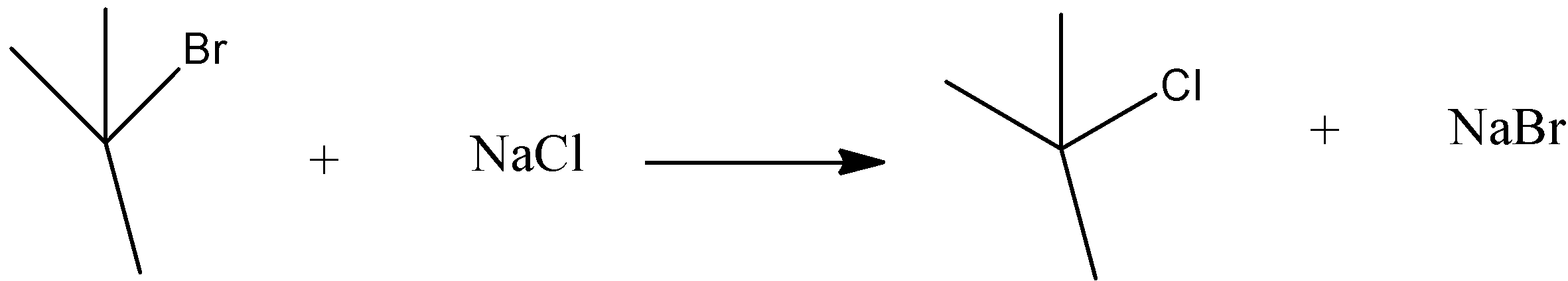
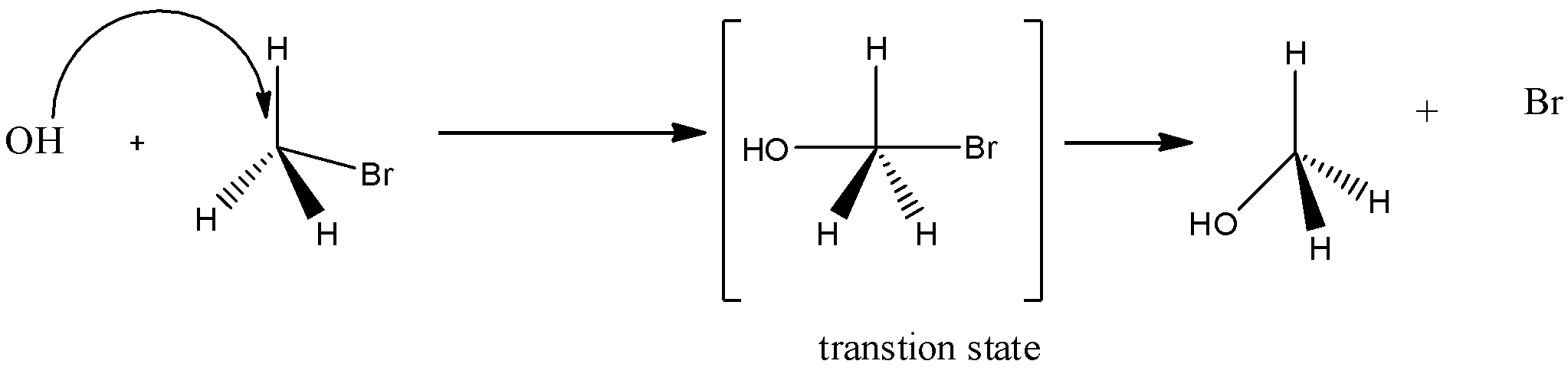
Option B) SN2 reactions are the one in which a transition state is formed. Rate of reaction depends on both the reactants given in the reaction and thus it is a bimolecular reaction. It shows inversion configuration thus it is said to be stereospecific.
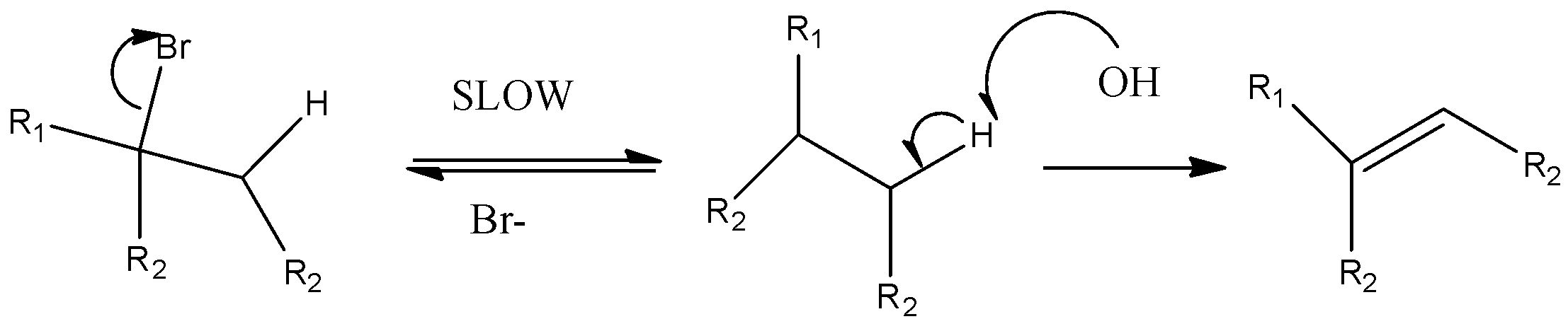
Option C) E1 reactions are non stereospecific reactions, this reaction leads to the formation of carbocation thus the reaction is unimolecular as rate depends only on one step. The reaction yields two products.
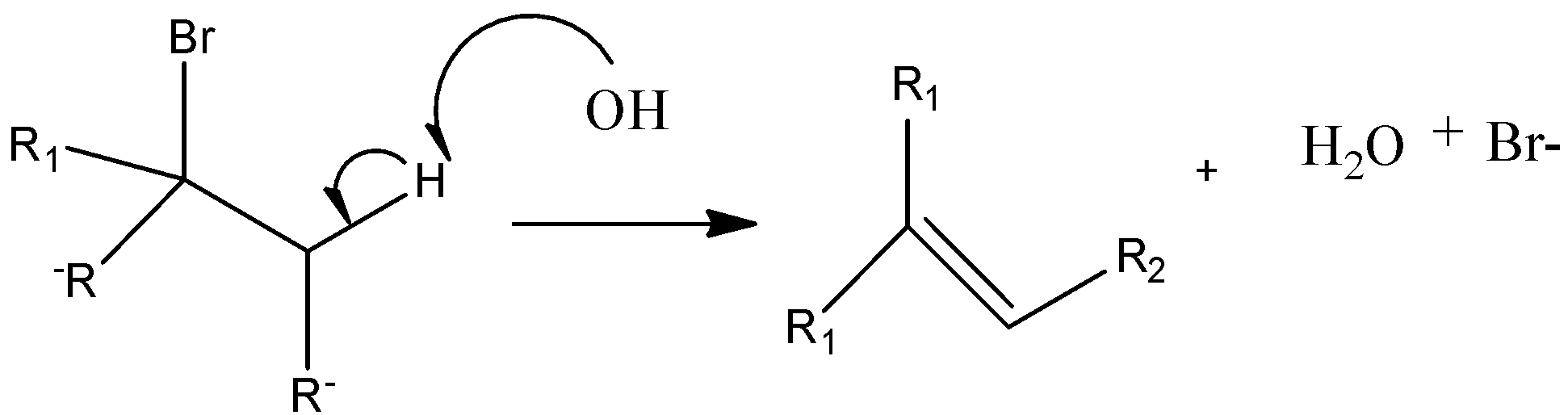
Option D) E2 reactions are stereospecific reactions as it has anti and syn products. The breaking and making of bonds takes place simultaneously, the reaction is said to be bimolecular as rate depends on concentration of reactants. The reaction is said to be stereoselective as the product is major.
Note:
We have to know that unimolecular reactions are the one in which only one reactant participates whereas in bimolecular reactions two reactants participate which are involved in a reaction. SN2 reactions are accompanied by inversion of configuration. In E2 ,the breaking and making of bonds takes place simultaneously.
Complete answer:
We will look at all the options to answer this question.
Option A) SN1 reactions are the one in which carbocation as an intermediate is formed and nucleophile can attack from both the positions, this reaction is unimolecular and rate depends only on the first step. So this reaction is non-stereospecific, thus this option is incorrect.

Option B) SN2 reactions are the one in which a transition state is formed. Rate of reaction depends on both the reactants given in the reaction and thus it is a bimolecular reaction. It shows inversion configuration thus it is said to be stereospecific.

Option C) E1 reactions are non stereospecific reactions, this reaction leads to the formation of carbocation thus the reaction is unimolecular as rate depends only on one step. The reaction yields two products.

Option D) E2 reactions are stereospecific reactions as it has anti and syn products. The breaking and making of bonds takes place simultaneously, the reaction is said to be bimolecular as rate depends on concentration of reactants. The reaction is said to be stereoselective as the product is major.

Note:
We have to know that unimolecular reactions are the one in which only one reactant participates whereas in bimolecular reactions two reactants participate which are involved in a reaction. SN2 reactions are accompanied by inversion of configuration. In E2 ,the breaking and making of bonds takes place simultaneously.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




