
Which of the following reactions are feasible?
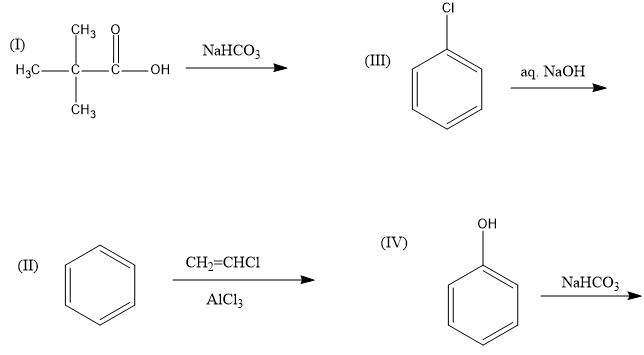
(A) $I,II$ and $IV$
(B) $I,II$ and $III$
(C) Only $III$
(D) Only $I$

Answer
497.4k+ views
Hint: To know whether a chemical reaction (organic reaction) is feasible or not, we need to know the structure of the reactants and their chemical properties. We should also know about the chemical properties of the reagents which are used in these reactions. Based on these properties, we will be able to know if a reaction is feasible or not.
Complete answer:
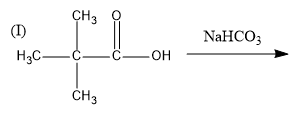
In this reaction, reactant is an acid and sodium bicarbonate $(NaHC{O_3})$ is a base. Therefore, it is an acid-base reaction and sodium salt of carboxylic acid will be formed. This reaction is feasible.
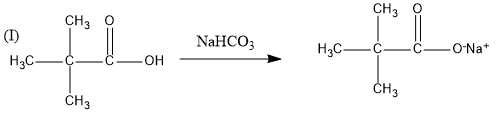
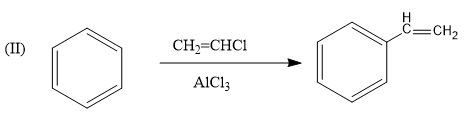
This reaction is known as the friedel crafts reaction in which a Lewis acid is added as a reagent which increases electron density on the benzene ring. Therefore, it is a feasible reaction and the reaction is given as follows:
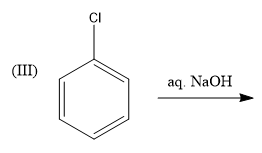
At normal temperature and pressure conditions, Chlorobenzene does not react with aqueous NaOH to form phenol because Chlorobenzene is very stable due to resonance. Hence, this reaction is not feasible.
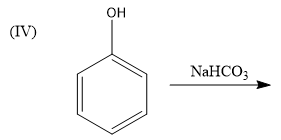
We know that sodium bicarbonate is a base and it reacts with acids to form a sodium salt. But phenol is weakly acidic in nature and hence, phenoxide ion (sodium salt of phenol) will not be formed in this reaction. Hence, this reaction is not feasible.
Therefore, the correct option is (C) Only $III$ .
Note:
We should remember that phenols are acidic in nature but they do not react with sodium bicarbonate to give sodium salt of phenol and a brisk effervescence. This is because phenols are not strong acids. So, based on the chemical properties of the substances we can determine the feasibility of any reaction.
Complete answer:

In this reaction, reactant is an acid and sodium bicarbonate $(NaHC{O_3})$ is a base. Therefore, it is an acid-base reaction and sodium salt of carboxylic acid will be formed. This reaction is feasible.


This reaction is known as the friedel crafts reaction in which a Lewis acid is added as a reagent which increases electron density on the benzene ring. Therefore, it is a feasible reaction and the reaction is given as follows:


At normal temperature and pressure conditions, Chlorobenzene does not react with aqueous NaOH to form phenol because Chlorobenzene is very stable due to resonance. Hence, this reaction is not feasible.

We know that sodium bicarbonate is a base and it reacts with acids to form a sodium salt. But phenol is weakly acidic in nature and hence, phenoxide ion (sodium salt of phenol) will not be formed in this reaction. Hence, this reaction is not feasible.
Therefore, the correct option is (C) Only $III$ .
Note:
We should remember that phenols are acidic in nature but they do not react with sodium bicarbonate to give sodium salt of phenol and a brisk effervescence. This is because phenols are not strong acids. So, based on the chemical properties of the substances we can determine the feasibility of any reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




