
Which of the following represents hexadentate ligand?
A.EDTA
B.DMG
C.Ethylenediamine
D.None of the above
Answer
565.2k+ views
Hint:Ligands are atoms or groups of ligands that bind to a central metal atom to form a coordination complex. The nature of this ligand-metal bond can be ionic or covalent. Some ligands can form more than one bond with the metal atom, these are known as polydentate ligands.
Complete step by step answer:
-Denticity refers to the number of donor groups in the single ligand that can form bonds with the central metal atom.
- Ligand which has only one donor atom is called monodentate ligands, ligands having two donor atoms are called a bidentate ligand.
-DMG stands for Dimethylglyoxime. It binds with metal atoms with two bonds one with each nitrogen atom. Hence it is a bidentate ligand.
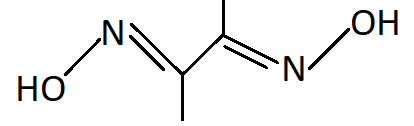
-Similar to DMG, ethylenediamine also has two nitrogen atoms as donor atoms and is a bidentate ligand.
${{{H}}_{{2}}}{{N - C}}{{{H}}_{{2}}}{{ - C}}{{{H}}_{{2}}}{{ - N}}{{{H}}_{{2}}}$
-Similarly, a ligand having six donor atoms is called a hexadentate ligand.
-EDTA refers to ethylenediaminetetraacetic acid. EDTA has two nitrogen atoms and four oxygen atoms as donor atoms. Thus it is a hexadentate ligand
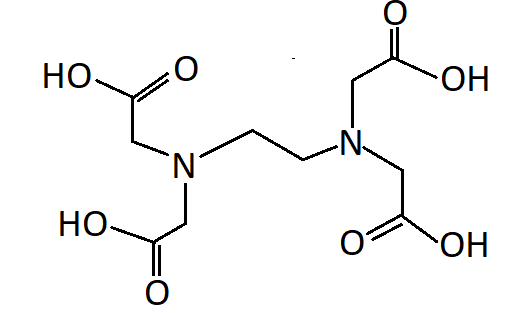
Hence the correct option is A. EDTA is a hexadentate ligand.
Note:
EDTA imparts extra stability to the coordination complex due to its chelate effect.
Chelation is the property of polydentate ligands which means ligands that have more than one donor atom.
When a polydentate ligand bonds with the central metal atom or ion, it forms a ring. This ring formation because of the presence of more than one donor atoms is called chelation.
The ring formation or chelation enhances the stability of the coordination complex as compared to the one having no chelation, this effect is known as the chelate effect.
Complete step by step answer:
-Denticity refers to the number of donor groups in the single ligand that can form bonds with the central metal atom.
- Ligand which has only one donor atom is called monodentate ligands, ligands having two donor atoms are called a bidentate ligand.
-DMG stands for Dimethylglyoxime. It binds with metal atoms with two bonds one with each nitrogen atom. Hence it is a bidentate ligand.

-Similar to DMG, ethylenediamine also has two nitrogen atoms as donor atoms and is a bidentate ligand.
${{{H}}_{{2}}}{{N - C}}{{{H}}_{{2}}}{{ - C}}{{{H}}_{{2}}}{{ - N}}{{{H}}_{{2}}}$
-Similarly, a ligand having six donor atoms is called a hexadentate ligand.
-EDTA refers to ethylenediaminetetraacetic acid. EDTA has two nitrogen atoms and four oxygen atoms as donor atoms. Thus it is a hexadentate ligand

Hence the correct option is A. EDTA is a hexadentate ligand.
Note:
EDTA imparts extra stability to the coordination complex due to its chelate effect.
Chelation is the property of polydentate ligands which means ligands that have more than one donor atom.
When a polydentate ligand bonds with the central metal atom or ion, it forms a ring. This ring formation because of the presence of more than one donor atoms is called chelation.
The ring formation or chelation enhances the stability of the coordination complex as compared to the one having no chelation, this effect is known as the chelate effect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




