
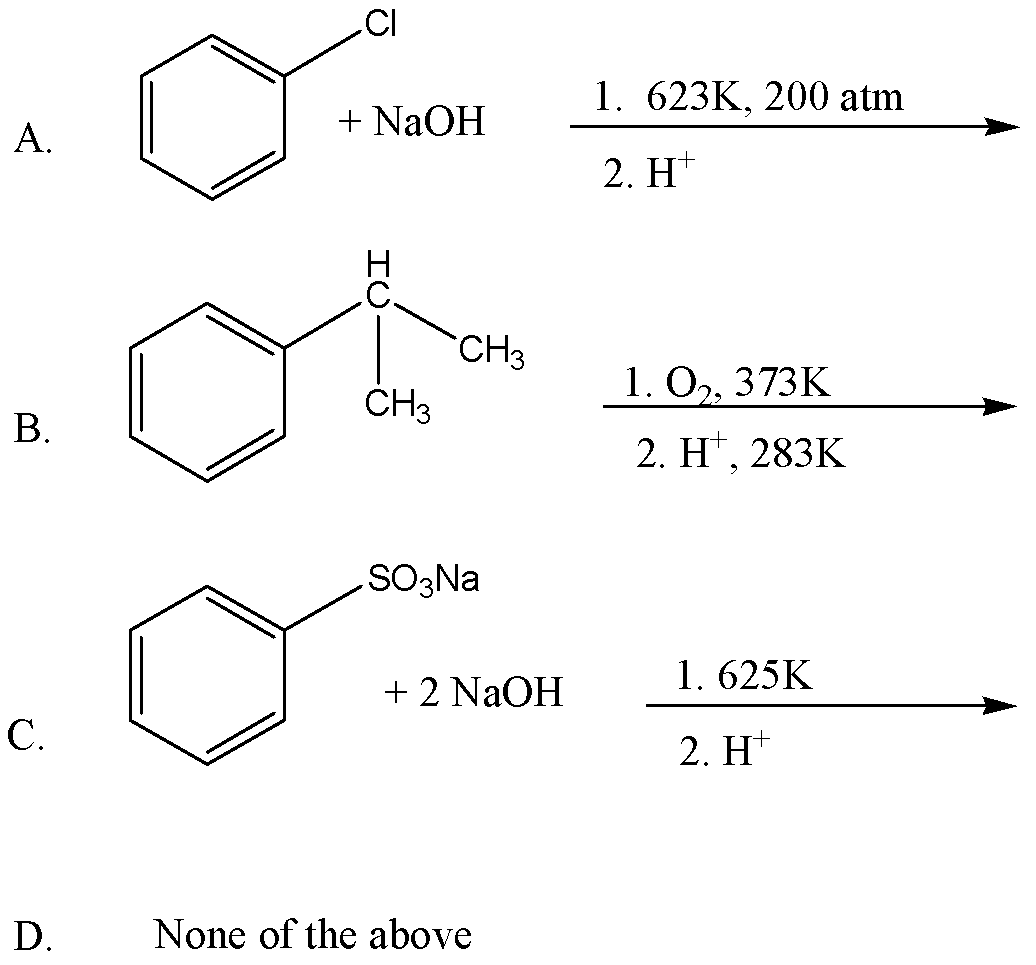
Which of the following represents the Dow process for the manufacture of phenol?

Answer
584.4k+ views
Hint: We must remember that the Chlorobenzene is an organic aromatic compound with the chemical formula\[{C_6}{H_5}Cl\]. This is colorless and due to its flammable property, it is a common solvent and a widely used intermediate to form a product.
Complete step by step solution:
We must understand that the Dow's Process is the hydrolysis of \[{C_6}{H_5}Cl\] (chlorobenzene) for the preparation of \[{C_6}{H_6}O\](phenol). When we react \[{C_6}{H_5}Cl\] (chlorobenzene) with aqueous \[\left( {NaOH} \right)\] solution it produces \[\left( {{C_6}{H_5}ONa} \right)\] sodium phenoxide and then phenol upon reacting with $H_+$.
In practical terms, we can prepare phenol from chlorobenzene when reacting with\[NaOH\], in particular two steps. First, Chlorobenzene is heated with 6-8% solution of sodium hydroxide at 623K under the pressure of 200 atm to form \[\left( {{C_6}{H_5}ONa} \right)\] Sodium Phenoxide. Then Sodium Phenoxide is acidified with dil. HCl (Hydrochloric Acid) to form \[{C_6}{H_6}O\] (Phenol).
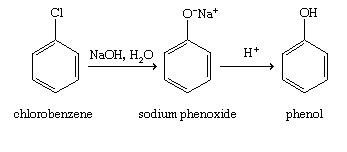
Therefore, the answer for the question is option A. :
Note: We can also use the Dow process (electrolytic method) to extract bromine from a highly concentrated \[NaCl\] solution known as brine.
In practical, bromide-containing high concentrations of Brines are treated with \[{H_2}S{O_4}\](sulphuric acid) and bleaching powder to oxidize Br− (bromide) to Br2 (bromine), which remains dissolved in the water. The aqueous solution is shifted into the sacking bag and water is passed through causing bromine to volatilize freely. Bromine is trapped with iron turns to give a solution of \[FeB{r_3}\](ferric bromide). Treatment with more iron metal converts the \[FeB{r_3}\] (ferric bromide) to \[FeB{r_2}\](ferrous bromide).
Complete step by step solution:
We must understand that the Dow's Process is the hydrolysis of \[{C_6}{H_5}Cl\] (chlorobenzene) for the preparation of \[{C_6}{H_6}O\](phenol). When we react \[{C_6}{H_5}Cl\] (chlorobenzene) with aqueous \[\left( {NaOH} \right)\] solution it produces \[\left( {{C_6}{H_5}ONa} \right)\] sodium phenoxide and then phenol upon reacting with $H_+$.
In practical terms, we can prepare phenol from chlorobenzene when reacting with\[NaOH\], in particular two steps. First, Chlorobenzene is heated with 6-8% solution of sodium hydroxide at 623K under the pressure of 200 atm to form \[\left( {{C_6}{H_5}ONa} \right)\] Sodium Phenoxide. Then Sodium Phenoxide is acidified with dil. HCl (Hydrochloric Acid) to form \[{C_6}{H_6}O\] (Phenol).

Therefore, the answer for the question is option A. :
Note: We can also use the Dow process (electrolytic method) to extract bromine from a highly concentrated \[NaCl\] solution known as brine.
In practical, bromide-containing high concentrations of Brines are treated with \[{H_2}S{O_4}\](sulphuric acid) and bleaching powder to oxidize Br− (bromide) to Br2 (bromine), which remains dissolved in the water. The aqueous solution is shifted into the sacking bag and water is passed through causing bromine to volatilize freely. Bromine is trapped with iron turns to give a solution of \[FeB{r_3}\](ferric bromide). Treatment with more iron metal converts the \[FeB{r_3}\] (ferric bromide) to \[FeB{r_2}\](ferrous bromide).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




