
Which of the following shapes $S{{F}_{4}}$ is more stable and why?
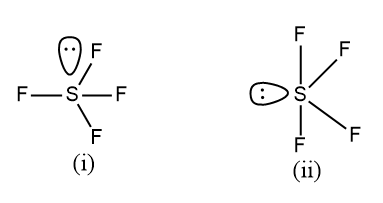
(a) Lone pair at axial position is stable
(b) Lone pair at equatorial position is stable
(c) Both are equally stable due to lp-bp repulsion
(d) Both are unstable since $S{{F}_{4}}$ has a tetrahedral shape
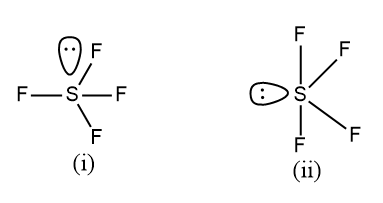
Answer
541.5k+ views
Hint: The force of repulsion between the lone pairs and bond pairs have a great effect in determining the shape of the molecule. The lone pair –lone pair interaction is the strongest and the bond pair-bond pair interaction is the least.
Complete answer:
So in the question, it is asked that, from the given two figures of $S{{F}_{4}}$, which will be the most stable one, we have to comment on the stability of two structures.
First, we have to compare the structures and the positions of the atoms arranged in space.
In one structure the lone pair is placed in the axial position and in the other, it is present in the equatorial position.
Now let's consider the stability of both structures concerning the force of repulsion present.
In the first structure, there are three fluorine atoms present in the vicinity which will experience the lone pair-bond pair repulsion force with the lone pair of Sulphur atom.
In the second structure, there are only two atoms of S present in the vicinity which will experience the lone pair-bond pair repulsion from the lone pair present in the Sulphur atom.
As we know that the structure with a minimum force of repulsion will have the maximum stability. Therefore, the structure (ii) will have the maximum stability since it possesses the least repulsion of lone pair-bond pair. And other types of interactions are the same for both structures.
Hence the correct answer for the above-given question is option (B), the lone pair at the equatorial position will be the stable one.
Note:
Students always get confused with the shapes of molecules, many write shape of $S{{F}_{4}}$ as tetrahedrally as it has four bond pairs but it also has a lone pair, because of which repulsive force of lone pair-bond pair comes in and have to change the arrangement of the atoms to minimize the repulsion and maximize the stability of the molecule.
And the magnitude of the force of repulsion will not be the same for the compounds which have the same atoms, since it differs from the arrangement of the atoms in space.
Complete answer:
So in the question, it is asked that, from the given two figures of $S{{F}_{4}}$, which will be the most stable one, we have to comment on the stability of two structures.
First, we have to compare the structures and the positions of the atoms arranged in space.
In one structure the lone pair is placed in the axial position and in the other, it is present in the equatorial position.
Now let's consider the stability of both structures concerning the force of repulsion present.
In the first structure, there are three fluorine atoms present in the vicinity which will experience the lone pair-bond pair repulsion force with the lone pair of Sulphur atom.
In the second structure, there are only two atoms of S present in the vicinity which will experience the lone pair-bond pair repulsion from the lone pair present in the Sulphur atom.
As we know that the structure with a minimum force of repulsion will have the maximum stability. Therefore, the structure (ii) will have the maximum stability since it possesses the least repulsion of lone pair-bond pair. And other types of interactions are the same for both structures.
Hence the correct answer for the above-given question is option (B), the lone pair at the equatorial position will be the stable one.
Note:
Students always get confused with the shapes of molecules, many write shape of $S{{F}_{4}}$ as tetrahedrally as it has four bond pairs but it also has a lone pair, because of which repulsive force of lone pair-bond pair comes in and have to change the arrangement of the atoms to minimize the repulsion and maximize the stability of the molecule.
And the magnitude of the force of repulsion will not be the same for the compounds which have the same atoms, since it differs from the arrangement of the atoms in space.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




