
Which of the following shows the structure of Neohexane?
A.
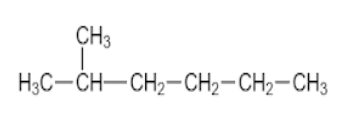
B.
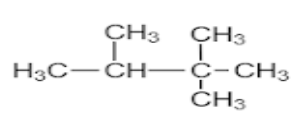
C.
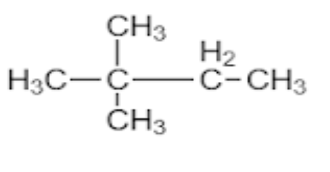
D.
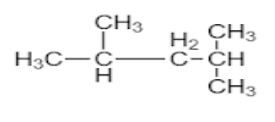




Answer
570.9k+ views
Hint: In this question, the chemical name of the compound is given. To obtain the molecular structure of the compound, we will need the IUPAC name to draw the parent chain and then the substituents.
Complete answer:
- Neohexane is an organic compound with the IUPAC name 2,2-Dimethylbutane.
- The prefix neo comes when a carbon atom is surrounded by other 4 carbons and hexane consists of total 6 carbon atoms. This can be easily explained by statements given below.
- In the organic compound the parent chain Is butane, which means it has four carbons in the main chain and it has “-ane” suffix which means all the carbon in the main chain have single bonds.
- Now, we name the substituents. Here, the substituent is methyl, where “meth” is a suffix which means there is one carbon in the chain of substituent.
- Dimethyl is used to show that the substituent is repeated at two positions, as ‘di’ means two.
- So, for checking the position of substituents here the numbering given is 2,2 which means both the substituents are placed on the 2nd carbon.
- The structure of Neohexane would be as shown below
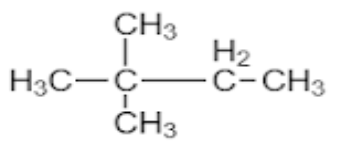
Therefore, the answer to the question is (C).
Note:
The rule of IUPAC naming and drawing structure from IUPAC naming is similar but in simple language we can say they are opposite. This is because, in IUPAC naming we start naming from the substituent but in the case of drawing structure from IUPAC naming we first see the main chain.
Complete answer:
- Neohexane is an organic compound with the IUPAC name 2,2-Dimethylbutane.
- The prefix neo comes when a carbon atom is surrounded by other 4 carbons and hexane consists of total 6 carbon atoms. This can be easily explained by statements given below.
- In the organic compound the parent chain Is butane, which means it has four carbons in the main chain and it has “-ane” suffix which means all the carbon in the main chain have single bonds.
- Now, we name the substituents. Here, the substituent is methyl, where “meth” is a suffix which means there is one carbon in the chain of substituent.
- Dimethyl is used to show that the substituent is repeated at two positions, as ‘di’ means two.
- So, for checking the position of substituents here the numbering given is 2,2 which means both the substituents are placed on the 2nd carbon.
- The structure of Neohexane would be as shown below

Therefore, the answer to the question is (C).
Note:
The rule of IUPAC naming and drawing structure from IUPAC naming is similar but in simple language we can say they are opposite. This is because, in IUPAC naming we start naming from the substituent but in the case of drawing structure from IUPAC naming we first see the main chain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




