
Which of the following silicates are nonplanar?
A. Single chain.
B. Double chain silicate.
C. 2D or sheet-like silicate.
D. Cyclic silicate.
Answer
563.7k+ views
Hint: Electronegativity is the tendency of an atom to attract the pair of electrons. Polarity of Molecules are related to the electronegativities of the atoms or the molecules. A molecule can be either a polar molecule, non-polar molecule or an ionic molecule.
Complete step by step answer:
Silicates are the minerals containing silicon and oxygen. The basic structural unit of a silicate mineral is the \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron. In a \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron each Silicon atom is covalently bonded to 4 oxygen atoms.
Silicon has an oxidation state of \[ + 4\] and Oxygen has an oxidation state of \[ - 2\].
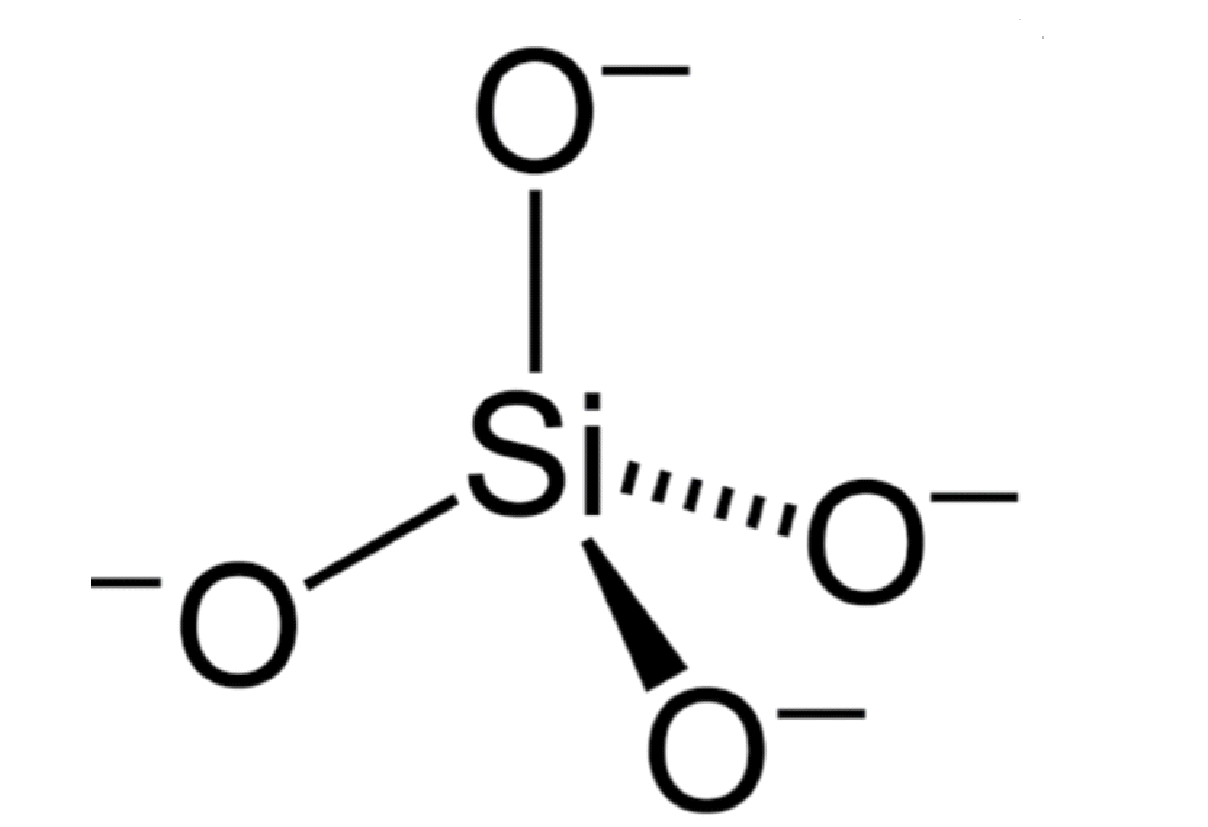
Therefore, the overall charge on the silicate tetrahedron \[ = {\text{ }} + 4{\text{ }} + {\text{ }}\left( {4{\text{ }} \times {\text{ }} - 2} \right){\text{ }} = {\text{ }} - 4\]
The \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron can be represented in many ways. The tetrahedra can link together by sharing corners forming \[{\text{Si - O - Si}}\] covalent bonds. A number of different structures are therefore possible.
The tetrahedra joined together to form rings, chains, bands and sheets.
Depending on the ways in which the tetrahedral units are linked, the silicates are classified different types some of these are, Orthosilicates, Pyro silicate, Cyclic silicates, Chain silicates, Double chain silicate, Sheet silicates, Three dimensional silicates
Single chain silicates: Here, each \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] shares two oxygen’s with two adjacent tetrahedrons. A long chain is formed.
Double chain silicates: In this silicates adjacent tetrahedrons share two of the oxygen’s to continue the chain, or three oxygen atoms to connect also to a second chain, giving rise to parallel double chains.
Sheet silicates: The general formula is \[\left( {{\text{Si2}}{{\text{O}}_{\text{5}}}} \right)_{\text{n}}^{{\text{2n - }}}\]. Here the \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron shares three oxygen atoms forming two-dimensional sheets. The silicates can be cleaved easily just like graphite. They have van der Waal's forces.
Cyclic silicates: They contain \[\left( {{\text{Si}}{{\text{O}}_{\text{3}}}} \right)_{\text{n}}^{{\text{2n - }}}\] ions, they are formed by three or more \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedral units linked cyclically. Every unit shares two oxygen atoms with every other unit.
All the above forms are not polar in nature.
So, the correct answer is Option A,B,C,D.
Additional information:
Some of the application of silicates are microchips, quartz crystals, concrete and masonry treatment, detergent auxiliaries, water treatment etc.
Note: Zeolites are aluminosilicate mainly used as adsorbents and catalysts. Structure is porous and can accommodate a wide variety of cations, such as \[{\text{Na + , K + , Ca2 + , Mg2 + }}\] and others. These positive ions are loosely held and can readily exchange.
Complete step by step answer:
Silicates are the minerals containing silicon and oxygen. The basic structural unit of a silicate mineral is the \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron. In a \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron each Silicon atom is covalently bonded to 4 oxygen atoms.
Silicon has an oxidation state of \[ + 4\] and Oxygen has an oxidation state of \[ - 2\].

Therefore, the overall charge on the silicate tetrahedron \[ = {\text{ }} + 4{\text{ }} + {\text{ }}\left( {4{\text{ }} \times {\text{ }} - 2} \right){\text{ }} = {\text{ }} - 4\]
The \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron can be represented in many ways. The tetrahedra can link together by sharing corners forming \[{\text{Si - O - Si}}\] covalent bonds. A number of different structures are therefore possible.
The tetrahedra joined together to form rings, chains, bands and sheets.
Depending on the ways in which the tetrahedral units are linked, the silicates are classified different types some of these are, Orthosilicates, Pyro silicate, Cyclic silicates, Chain silicates, Double chain silicate, Sheet silicates, Three dimensional silicates
Single chain silicates: Here, each \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] shares two oxygen’s with two adjacent tetrahedrons. A long chain is formed.
Double chain silicates: In this silicates adjacent tetrahedrons share two of the oxygen’s to continue the chain, or three oxygen atoms to connect also to a second chain, giving rise to parallel double chains.
Sheet silicates: The general formula is \[\left( {{\text{Si2}}{{\text{O}}_{\text{5}}}} \right)_{\text{n}}^{{\text{2n - }}}\]. Here the \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedron shares three oxygen atoms forming two-dimensional sheets. The silicates can be cleaved easily just like graphite. They have van der Waal's forces.
Cyclic silicates: They contain \[\left( {{\text{Si}}{{\text{O}}_{\text{3}}}} \right)_{\text{n}}^{{\text{2n - }}}\] ions, they are formed by three or more \[{\text{SiO}}_{\text{4}}^{{\text{4 - }}}\] tetrahedral units linked cyclically. Every unit shares two oxygen atoms with every other unit.
All the above forms are not polar in nature.
So, the correct answer is Option A,B,C,D.
Additional information:
Some of the application of silicates are microchips, quartz crystals, concrete and masonry treatment, detergent auxiliaries, water treatment etc.
Note: Zeolites are aluminosilicate mainly used as adsorbents and catalysts. Structure is porous and can accommodate a wide variety of cations, such as \[{\text{Na + , K + , Ca2 + , Mg2 + }}\] and others. These positive ions are loosely held and can readily exchange.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




