
Which of the following statements about a weak acid strong base titration is/ are correct?
(A) The PH after the equivalence point of the weak point of the weak acid strong base titration is determined by using the ${K_b}$ expression for the conjugate base.
(B) A buffer solution of weak acid and its conjugate base is formed before the equivalence is reached.
(C) The pH at the equivalence point of a weak acid monoprotic acid strong base titration is equal to pH at equivalence point of a strong acid-strong base titration.
(D) The increase in pH in the region near the equivalence. The increase in pH in the region near the equivalence point of a weak acid strong base titration is greater than the pH change in the same region of a strong base titration.
Answer
577.5k+ views
Hint: To solve this, firstly we should know what weak acid and strong base are. The acid- base titration is a method of quantitative analysis where the concentration of acid and base are determined by neutralization. The neutralization is done by adding the standard volume of acid and base of known concentrations.
Complete answer:
The acid base titration is accomplished by pH. A plot is drawn between the pH of the solution during titration and the acid from the burette is known as titration curve.
Acetic acid is a weak acid and sodium hydroxide is a strong base.
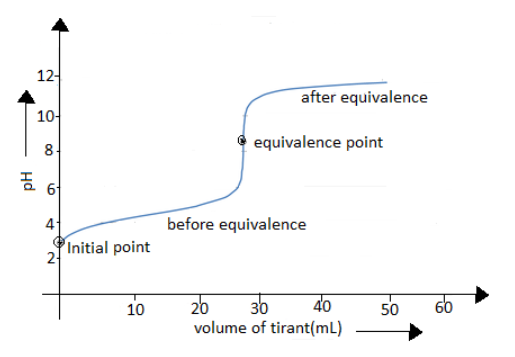
Observation from graph:
-At initial point it increases sharply up to a certain point. This increase is due to the anion in weak acid which acts as the common ion that reduces the ionization of the acid.
\[C{H_3}COOH + [N{a^ + } + O{H^ - }] \to C{H_3}CO{O^ - } + N{a^ + } + {H_2}O\]
- There will be a sharp increase in the beginning, but after a point it gradually changes. This change is due to the formation of the buffer solution of weak acid and its conjugate base, before the equivalence is reached.
-The equivalence point is the point where the acid and base are neutralized. Here, the concentration of the weak acid is equal to the concentration of its conjugate base. Therefore the pH=pKa. pH=pKa, which can also be written as pH=${K_b}$. The pH will be almost 7 or above where all the acid is converted to its conjugate base.
-After reaching the equivalence point, it moves backward.
From the above discussion we can conclude that option A and B are the correct answer.
Note:
Understand the graph and try to know the reason behind each change occurred. This will help you to solve questions of this type. The change in curve due to the formation of buffer solution will continue until the base overcomes the buffer formed.
Complete answer:
The acid base titration is accomplished by pH. A plot is drawn between the pH of the solution during titration and the acid from the burette is known as titration curve.
Acetic acid is a weak acid and sodium hydroxide is a strong base.

Observation from graph:
-At initial point it increases sharply up to a certain point. This increase is due to the anion in weak acid which acts as the common ion that reduces the ionization of the acid.
\[C{H_3}COOH + [N{a^ + } + O{H^ - }] \to C{H_3}CO{O^ - } + N{a^ + } + {H_2}O\]
- There will be a sharp increase in the beginning, but after a point it gradually changes. This change is due to the formation of the buffer solution of weak acid and its conjugate base, before the equivalence is reached.
-The equivalence point is the point where the acid and base are neutralized. Here, the concentration of the weak acid is equal to the concentration of its conjugate base. Therefore the pH=pKa. pH=pKa, which can also be written as pH=${K_b}$. The pH will be almost 7 or above where all the acid is converted to its conjugate base.
-After reaching the equivalence point, it moves backward.
From the above discussion we can conclude that option A and B are the correct answer.
Note:
Understand the graph and try to know the reason behind each change occurred. This will help you to solve questions of this type. The change in curve due to the formation of buffer solution will continue until the base overcomes the buffer formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




