
Which of the following statements is/are correct about the following reactions?
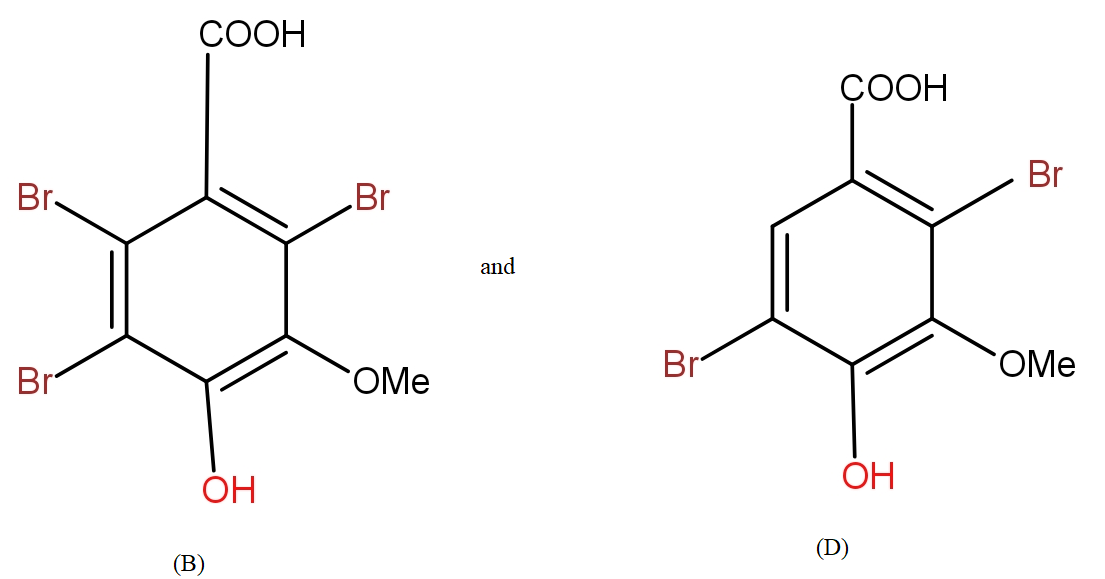
a.) The products (B) and (D) respectively, are :

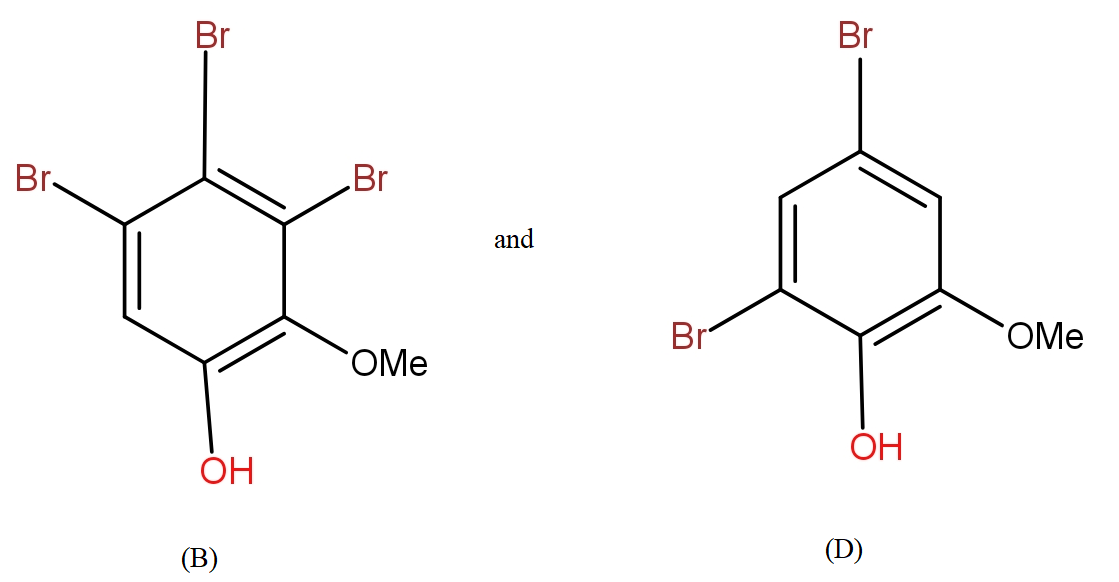
b.) The products (B) and (D) respectively, are :
c.) Phenol can not be chlorinated because the ring is susceptible to oxidation by $C{l_2}$.
d.) The formation of (C ) from (B) proceeds by Arsn reaction (addition – elimination).




Answer
593.4k+ views
Hint : The reaction is about bromination of substituted benzoic acid. When we add bromine water to 3-methoxy-4-hydroxy benzoic acid, Bromine atom gets substituted on the benzene ring in accordance with the carboxylic group.
Complete answer :
Let us move step by step to the answer.
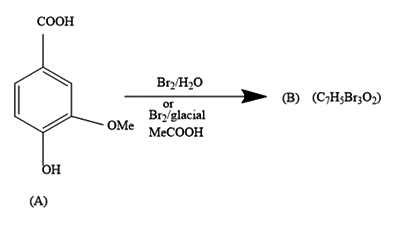
In the first reaction, we are given a reaction in which bromine water is added to 3-methoxy-4-hydroxy benzoic acid. The product formed contains seven carbon atoms, five hydrogen atoms, three bromine atoms and two oxygen atoms.
It comes under chemical reactions of carbonyl compounds. As we all know benzene is much stable due to resonance that it does not undergo addition reactions. It undergoes a substitution reaction in which H atom gets replaced by the incoming group.
The COOH group being the most dominating will decide the substitution of bromine atoms.
Further, from the options only, it is clearly visible that the compound in option a.) contains the equal number of atoms of each type as given in question in product (B). The molecular formula supports the answer a.).
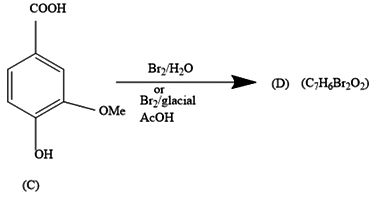
Even for the second reaction, the option a.) shows an equal number of atoms.
So, the option a.) is the correct answer.
Note :
The COOH group is meta directing in nature. So, it will direct the incoming bromine atoms to its meta position. While the OH and OMe groups are ortho and para directing in nature. In that option b.) could also be the answer but here molecular formula does not support it.
Further, decarboxylation with bromine water is uncommon since it is an oxidising agent.
Complete answer :
Let us move step by step to the answer.
In the first reaction, we are given a reaction in which bromine water is added to 3-methoxy-4-hydroxy benzoic acid. The product formed contains seven carbon atoms, five hydrogen atoms, three bromine atoms and two oxygen atoms.
It comes under chemical reactions of carbonyl compounds. As we all know benzene is much stable due to resonance that it does not undergo addition reactions. It undergoes a substitution reaction in which H atom gets replaced by the incoming group.
The COOH group being the most dominating will decide the substitution of bromine atoms.
Further, from the options only, it is clearly visible that the compound in option a.) contains the equal number of atoms of each type as given in question in product (B). The molecular formula supports the answer a.).
Even for the second reaction, the option a.) shows an equal number of atoms.
So, the option a.) is the correct answer.

Note :
The COOH group is meta directing in nature. So, it will direct the incoming bromine atoms to its meta position. While the OH and OMe groups are ortho and para directing in nature. In that option b.) could also be the answer but here molecular formula does not support it.
Further, decarboxylation with bromine water is uncommon since it is an oxidising agent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




