
Which of the following statements(s) about cyclohexane is/are correct?
A.Stability of different conformations of cyclohexane is $Chair > Boat > Twist{\text{ }}Boat > Half{\text{ }}Chair$
B.Only the chair form is free from angle strain.
C. Half chair has five C atoms in one plane and one C atom out of the plane. Hence, it has both eclipsing and bond angle strains and is the least stable conformer of cyclohexane
D.Twisting the boat to the skew boat conformation moves the flagpole H atoms away from each other and reduces the eclipsing strain. Hence, the twist boat is more stable than the boat conformation.
Answer
577.5k+ views
Hint: The different arrangement of atoms in space which is obtained due to free rotation about carbon-carbon single bonds is called conformation. Cyclohexane forms four conformations – chair, half chair, boat and twist boat.
Complete step by step answer:
Cyclohexane molecules have bond angle $120^0$ so it is expected to be reactive and strained but it is stable. This is because the molecule avoids strain by assuming conformation in which all the bond angles between carbon atoms are close to the tetrahedral angle of $109.5^0 $. So cyclohexane is said to be a non-planar molecule. It has four conformations- chair, boat, twist boat and half chair. The structures are given as-
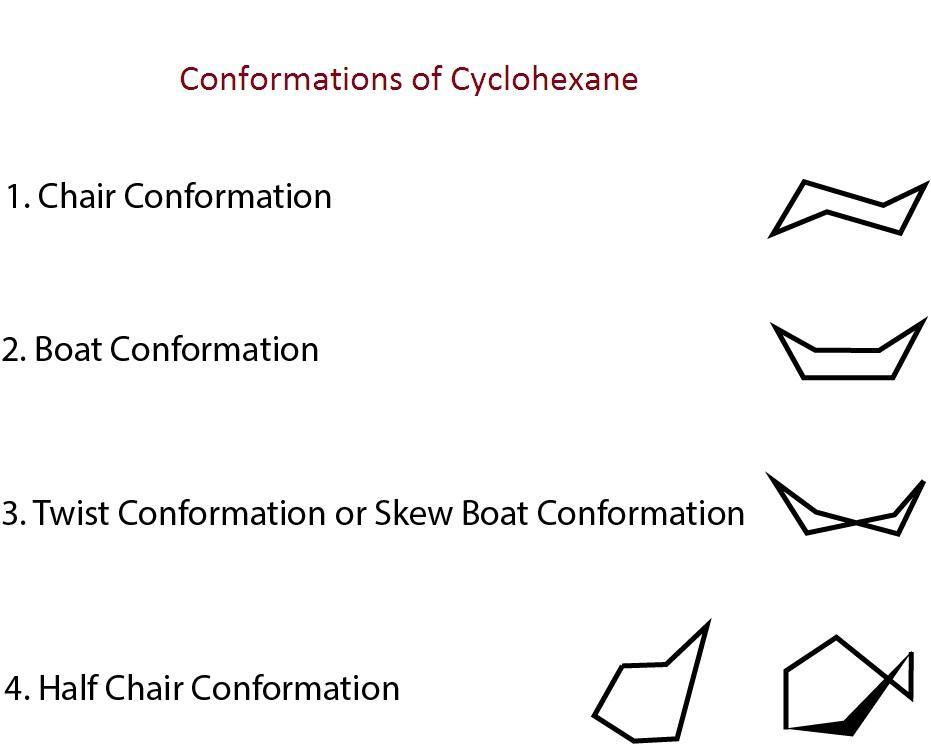
In chair conformation, the adjacent hydrogen atoms on all the neighboring carbon atoms are staggered and the force of repulsion in them is the minimum. It also does not have any angle strain. So it is most stable.
The half chair form is least stable as it has maximum strain as five carbon atoms are in one plane and one carbon atom is out of plane.
The boat conformation has steric strain due to interaction between two flagpole hydrogen and torsional strain is also present. It also does not have any angle strain. So it is less stable than a chair and twist boat.
Twist boat has less steric strain and torsional strain then the boat conformation due to which flagpole hydrogen atoms move away from each other and reduce the strain so it is more stable than boat conformation.
The stability order of different conformations of cyclohexane is $Chair > Twist{\text{ }}Boat > Boat > Half{\text{ }}Chair$
The correct options are option C and D.
Note:
The two hydrogen atoms bonded to first carbon and fourth carbon atoms in boat conformations are close and repel each other. These are called flagpole hydrogen. In boat conformation, they are close boats when we twist the boat conformation the flagpole hydrogen moves away from each other and we get skew boat or twist boat conformation.
Complete step by step answer:
Cyclohexane molecules have bond angle $120^0$ so it is expected to be reactive and strained but it is stable. This is because the molecule avoids strain by assuming conformation in which all the bond angles between carbon atoms are close to the tetrahedral angle of $109.5^0 $. So cyclohexane is said to be a non-planar molecule. It has four conformations- chair, boat, twist boat and half chair. The structures are given as-

In chair conformation, the adjacent hydrogen atoms on all the neighboring carbon atoms are staggered and the force of repulsion in them is the minimum. It also does not have any angle strain. So it is most stable.
The half chair form is least stable as it has maximum strain as five carbon atoms are in one plane and one carbon atom is out of plane.
The boat conformation has steric strain due to interaction between two flagpole hydrogen and torsional strain is also present. It also does not have any angle strain. So it is less stable than a chair and twist boat.
Twist boat has less steric strain and torsional strain then the boat conformation due to which flagpole hydrogen atoms move away from each other and reduce the strain so it is more stable than boat conformation.
The stability order of different conformations of cyclohexane is $Chair > Twist{\text{ }}Boat > Boat > Half{\text{ }}Chair$
The correct options are option C and D.
Note:
The two hydrogen atoms bonded to first carbon and fourth carbon atoms in boat conformations are close and repel each other. These are called flagpole hydrogen. In boat conformation, they are close boats when we twist the boat conformation the flagpole hydrogen moves away from each other and we get skew boat or twist boat conformation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




