
Which of the following undergoes aldol condensation

A.
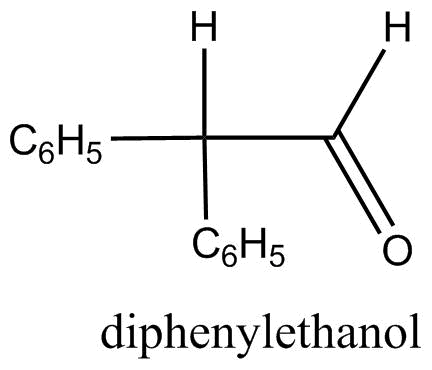
B.
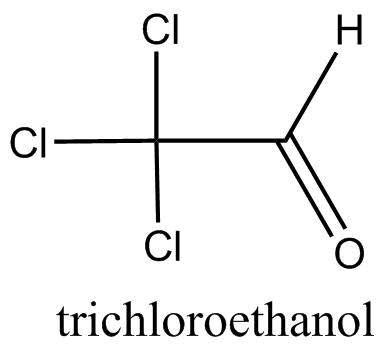
C.
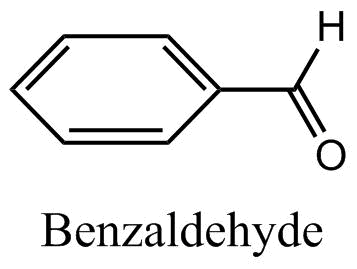
D.
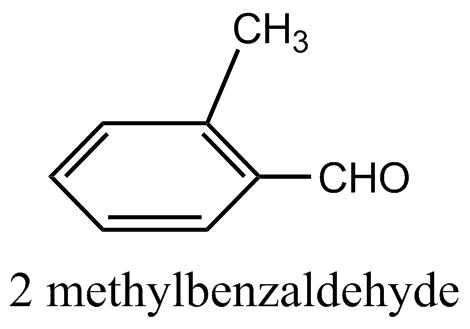





Answer
559.2k+ views
Hint: Consider searching for alpha hydrogen in aldol condensation. Aldol condensation can be easily understood as the addition of ketone or aldehyde within themselves or by cross-interaction, which yields $\beta - $hydroxy carbonyl.
Complete step by step answer:
Aldol condensation is a type of reaction where carbon-carbon bonds are formed when two carbonyl groups react with each other. The reaction results in the formation of $\beta - $ hydroxy carbonyl, which is known as aldol [aldehyde+ketone].
Now, in the formation of aldol condensation, a very important aspect is alpha hydrogen $[\alpha - H]$.
In chemistry we are introduced with the concept of alpha carbon and alpha hydrogen, the alpha carbon $[\alpha - C]$ in organic chemistry is referred to as the carbon atom attached to the functional group, such as the carboxylic group. Now, the hydrogen attached to alpha carbon is known as alpha hydrogen.
Now in the given options in the questions, we first need to find out which of the options has $[\alpha - H]$ , because without alpha hydrogen we cannot proceed further.
Diphenylethanal: By referring the structure in the question, we get to know that there is one alpha hydrogen in diphenyl ethanal. Therefore, aldol condensation can occur in it.
Trichloro ethanol: By referring to the structure in the question, we get to know that there is no alpha hydrogen in trichloro ethanal. Therefore, alcohol condensation cannot occur in it.
Benzaldehyde: By referring to the structure in the question, we get to know that there is no alpha hydrogen in benzaldehyde: Therefore, aldol condensation cannot occur in it.
2-methyl benzaldehyde: By referring to the structure in the question, we get to know that there is no alpha hydrogen in 2-methyl benzaldehyde. Therefore, alcohol condensation cannot occur in it.
Therefore, (A) Diphenyl ethanal is the only compound which will undergo aldol condensation.
Note: Before going to the complete conversion of the options given in the question, always remember and try to simplify the structure and note their nomenclature which will help you to avoid any further confusion.
Complete step by step answer:
Aldol condensation is a type of reaction where carbon-carbon bonds are formed when two carbonyl groups react with each other. The reaction results in the formation of $\beta - $ hydroxy carbonyl, which is known as aldol [aldehyde+ketone].
Now, in the formation of aldol condensation, a very important aspect is alpha hydrogen $[\alpha - H]$.
In chemistry we are introduced with the concept of alpha carbon and alpha hydrogen, the alpha carbon $[\alpha - C]$ in organic chemistry is referred to as the carbon atom attached to the functional group, such as the carboxylic group. Now, the hydrogen attached to alpha carbon is known as alpha hydrogen.
Now in the given options in the questions, we first need to find out which of the options has $[\alpha - H]$ , because without alpha hydrogen we cannot proceed further.
Diphenylethanal: By referring the structure in the question, we get to know that there is one alpha hydrogen in diphenyl ethanal. Therefore, aldol condensation can occur in it.
Trichloro ethanol: By referring to the structure in the question, we get to know that there is no alpha hydrogen in trichloro ethanal. Therefore, alcohol condensation cannot occur in it.
Benzaldehyde: By referring to the structure in the question, we get to know that there is no alpha hydrogen in benzaldehyde: Therefore, aldol condensation cannot occur in it.
2-methyl benzaldehyde: By referring to the structure in the question, we get to know that there is no alpha hydrogen in 2-methyl benzaldehyde. Therefore, alcohol condensation cannot occur in it.
Therefore, (A) Diphenyl ethanal is the only compound which will undergo aldol condensation.
Note: Before going to the complete conversion of the options given in the question, always remember and try to simplify the structure and note their nomenclature which will help you to avoid any further confusion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




