
Which of the following will be the most stable diazonium salt \[RN_{2}^{+}{{X}^{-}}\]?
A. \[C{{H}_{3}}N_{2}^{+}{{X}^{-}}\]
B. \[{{C}_{6}}{{H}_{5}}N_{2}^{+}{{X}^{-}}\]
C. \[C{{H}_{3}}C{{H}_{2}}N_{2}^{+}{{X}^{-}}\]
D. \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}N_{2}^{+}{{X}^{-}}\]
Answer
604.8k+ views
Hint: To answer this question we should know that resonance stabilisation occurs between the benzene nucleus and N-atom. And this resonance stabilisation provides stability. And in diazonium salts other than aryl one, there is no dispersal of positive charge because there is no resonance.
Complete step-by-step answer:
To answer this question, we should one by one look at the options. We have to draw the structures of every option.
First is \[C{{H}_{3}}N_{2}^{+}{{X}^{-}}\]
We can observe that there is an alkyl group attached to diazonium ions. And we know that there is no resonance occurrence in this structure. And by this we can say that this diazonium salt will have less stabilization.
So, now we will take the second option.
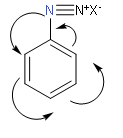
In the above structure we observe that diazonium ion is directly attached to the benzene ring. One more point to note is that there will be an occurrence of resonance in the benzene ring. So, from this we can say that this diazonium salt will be more stable because there dispersal of positive charge in the benzene ring due to resonance.
Now, we will take the third option.
\[C{{H}_{3}}C{{H}_{2}}N_{2}^{+}{{X}^{-}}\]: This diazonium salt will not be stable, because there will not be any resonance in this structure. And due to lack of resonance, the positive charge will not get dispersed. And due to all these reasons, this diazonium salt lacks stability.
Now, we should check the fourth option.

We observe that in this diazonium salt, the diazonium ion is attached to the methyl group. There will be no dispersal of positive charge and thus there will be no stabilization. So, there will be no stability in this diazonium salt.
From the above discussion, we got to know that the second option is correct. In the second option, the structure fulfils all the criteria to have stability. So, that’s option B will be the most stable diazonium salt.
Note: We should know about properties of diazonium salts and these are as follows:
They are ionic in nature.
They are water-soluble.
Aryl diazonium salts are colourless crystalline solids.
Benzenediazonium chloride is soluble in water but reacts with it only when warmed.
Benzenediazonium fluoroborate is not soluble in water. It is pretty stable at room temperature.
Complete step-by-step answer:
To answer this question, we should one by one look at the options. We have to draw the structures of every option.
First is \[C{{H}_{3}}N_{2}^{+}{{X}^{-}}\]
We can observe that there is an alkyl group attached to diazonium ions. And we know that there is no resonance occurrence in this structure. And by this we can say that this diazonium salt will have less stabilization.
So, now we will take the second option.

In the above structure we observe that diazonium ion is directly attached to the benzene ring. One more point to note is that there will be an occurrence of resonance in the benzene ring. So, from this we can say that this diazonium salt will be more stable because there dispersal of positive charge in the benzene ring due to resonance.
Now, we will take the third option.
\[C{{H}_{3}}C{{H}_{2}}N_{2}^{+}{{X}^{-}}\]: This diazonium salt will not be stable, because there will not be any resonance in this structure. And due to lack of resonance, the positive charge will not get dispersed. And due to all these reasons, this diazonium salt lacks stability.
Now, we should check the fourth option.

We observe that in this diazonium salt, the diazonium ion is attached to the methyl group. There will be no dispersal of positive charge and thus there will be no stabilization. So, there will be no stability in this diazonium salt.
From the above discussion, we got to know that the second option is correct. In the second option, the structure fulfils all the criteria to have stability. So, that’s option B will be the most stable diazonium salt.
Note: We should know about properties of diazonium salts and these are as follows:
They are ionic in nature.
They are water-soluble.
Aryl diazonium salts are colourless crystalline solids.
Benzenediazonium chloride is soluble in water but reacts with it only when warmed.
Benzenediazonium fluoroborate is not soluble in water. It is pretty stable at room temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




