
Which of the following will best convert nitrobenzene into 3-fluorobromobenzene?

A. $\dfrac{{{F_2}}}{{AlC{l_3}}}$, Zn/HCl , $\dfrac{{NaN{o_2}}}{{HCl}} - 0^\circ C$ , CuBr
B. $\dfrac{{B{r_2}}}{{FeB{r_3}}}$ ,$\dfrac{{SnC{l_2}}}{{HCl}}$. , $\dfrac{{NaN{o_2}}}{{HB{F_4}}} -
0^\circ C$ , heat
C. $\dfrac{{SnC{l_2}}}{{\;HCl}}$ , $\dfrac{{NaN{o_2}}}{{HB{F_4}}} - 0^\circ C$ , heat,
$\dfrac{{B{r_2}}}{{FeB{r_3}}}$
D. $\dfrac{{SnC{l_2}}}{{HCl}}$ , $\dfrac{{B{r_2}}}{{FeB{r_3}}}$ , $\dfrac{{NaN{o_2}}}{{HB{F_4}}} -
0^\circ C$ , heat

Answer
579.9k+ views
Hint: Nitrobenzene is brominated to form 1-bromo-3-nitrobenzene. And then the Nitro group is reduced to an amino group to obtain 3-bromoaniline. The amino group is diazotized and further replaced by Fluorine (F) to obtain 3-fluorobromobenzene.
Complete step by step answer:
Nitrobenzene is an organic compound with the chemical formula \[{C_6}{H_5}N{O_2}\] . It is a water insoluble pale-yellow oil with an almond-like odour. The best way to convert nitrobenzene into 3-fluorobromobenzene is:
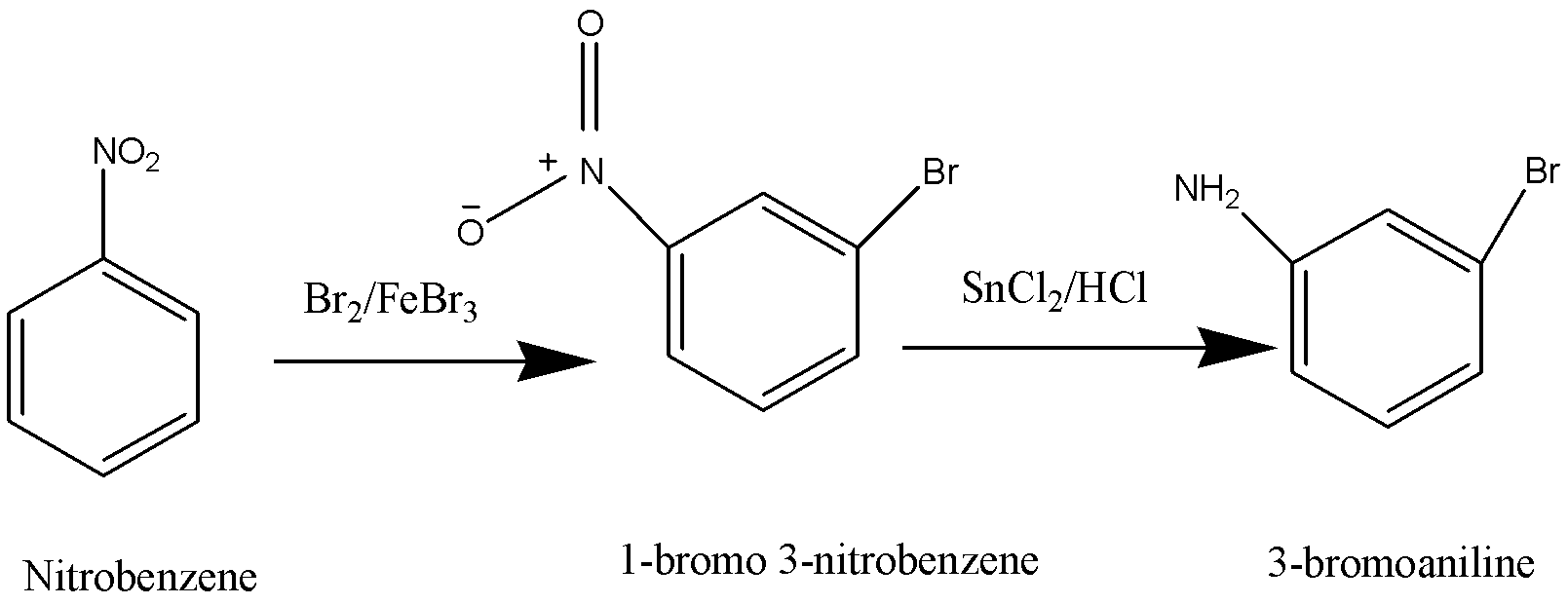

Nitrobenzene is brominated to form 1-bromo-3-nitrobenzene. Nitro group is then reduced to amino group to obtain 3 – bromoaniline. The amino group is then diazotized and it is then replaced by a Fluorine (F) so that we obtain 1-bromo-3-fluorobenzene or 3-fluorobromobenzene. Thus, the reagents which is given in the option B are used to make 3-
fluorobromobenzene.
Therefore, the correct option is (B).
Note: 3-fluorobromobenzene is a clear colourless light yellow. The molecular formula of 3-fluorobromobenzene or 1-bromo-3-fluorobenzene is ${C_6}{H_4}BrF$ . Its molecular weight is 175 g / mol. Its melting point is \[ - {8^\circ }C\] and its boiling point is \[149 - {151^\circ }C\] (lit). Its flash point is \[{102^\circ }F\] . Its density is 1.567 g / mL at \[{25^\circ }C\] (lit). It is insoluble in water. Its solubility is 0.4 g / l. It is liquid in form. It is used as an Intermediate of Liquid Crystals.
Complete step by step answer:
Nitrobenzene is an organic compound with the chemical formula \[{C_6}{H_5}N{O_2}\] . It is a water insoluble pale-yellow oil with an almond-like odour. The best way to convert nitrobenzene into 3-fluorobromobenzene is:


Nitrobenzene is brominated to form 1-bromo-3-nitrobenzene. Nitro group is then reduced to amino group to obtain 3 – bromoaniline. The amino group is then diazotized and it is then replaced by a Fluorine (F) so that we obtain 1-bromo-3-fluorobenzene or 3-fluorobromobenzene. Thus, the reagents which is given in the option B are used to make 3-
fluorobromobenzene.
Therefore, the correct option is (B).
Note: 3-fluorobromobenzene is a clear colourless light yellow. The molecular formula of 3-fluorobromobenzene or 1-bromo-3-fluorobenzene is ${C_6}{H_4}BrF$ . Its molecular weight is 175 g / mol. Its melting point is \[ - {8^\circ }C\] and its boiling point is \[149 - {151^\circ }C\] (lit). Its flash point is \[{102^\circ }F\] . Its density is 1.567 g / mL at \[{25^\circ }C\] (lit). It is insoluble in water. Its solubility is 0.4 g / l. It is liquid in form. It is used as an Intermediate of Liquid Crystals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




