
Which of the following will produce cyclic ketone on heating?
(A) $ C{H_2}{(COOH)_2} $
(B)
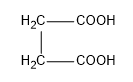
(C)
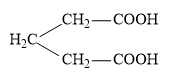
(D)
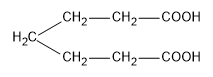
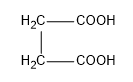

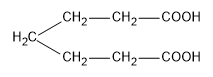
Answer
546.6k+ views
Hint: Cyclic ketone when formed then carbon dioxide gas is removed which means the compound will lose one carbon atom, so then we will see if the ring formed will be stable or not.
Complete Step by step answer:
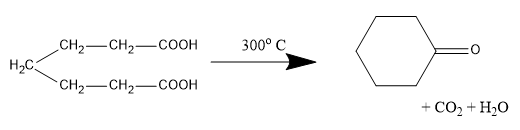
In the potions we are given dicarboxylic acids which have highly acid hydrogens and so, these groups are capable of getting reactions within themselves. Acids in which the two carboxylic groups are separated by some alkyl groups are close enough then we have the possibility of them interacting with each other and these reactions occur when the dicarboxylic are heated. When dicarboxylic acids are heated they have a tendency to undergo cyclization to form cyclic ketone. But for cyclization to happen the ring formed should not have strain which means that five and six membered rings are the most favored because of their least angle strain. Now in our question the first compound cannot undergo cyclization because a ring of two carbon atoms is not possible. Now, in the next compound (b) if the cyclization happens the ring formed will be a three membered ring which will be highly unstable due to angle strain, so this cyclization is not possible. In case of compound (c) on cyclization a four membered ring will be formed which again will be highly unstable due to angle strain, so this cyclization is also not possible. Now the only left is compound (d) which on cyclization will give a six membered ring which is stable, so this compound is capable of undergoing cyclization and will give cyclic ketone as shown below:
Note:
While in case of formation of cyclic ketone it should be noted that there is loss of one carbon atom. So, if we have an $ n $ number of carbon atoms then on cyclization the ring will have $ n - 1 $ carbon atoms.
Complete Step by step answer:
In the potions we are given dicarboxylic acids which have highly acid hydrogens and so, these groups are capable of getting reactions within themselves. Acids in which the two carboxylic groups are separated by some alkyl groups are close enough then we have the possibility of them interacting with each other and these reactions occur when the dicarboxylic are heated. When dicarboxylic acids are heated they have a tendency to undergo cyclization to form cyclic ketone. But for cyclization to happen the ring formed should not have strain which means that five and six membered rings are the most favored because of their least angle strain. Now in our question the first compound cannot undergo cyclization because a ring of two carbon atoms is not possible. Now, in the next compound (b) if the cyclization happens the ring formed will be a three membered ring which will be highly unstable due to angle strain, so this cyclization is not possible. In case of compound (c) on cyclization a four membered ring will be formed which again will be highly unstable due to angle strain, so this cyclization is also not possible. Now the only left is compound (d) which on cyclization will give a six membered ring which is stable, so this compound is capable of undergoing cyclization and will give cyclic ketone as shown below:

Note:
While in case of formation of cyclic ketone it should be noted that there is loss of one carbon atom. So, if we have an $ n $ number of carbon atoms then on cyclization the ring will have $ n - 1 $ carbon atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




