
Which of the following will react with $NaOI$?
A.
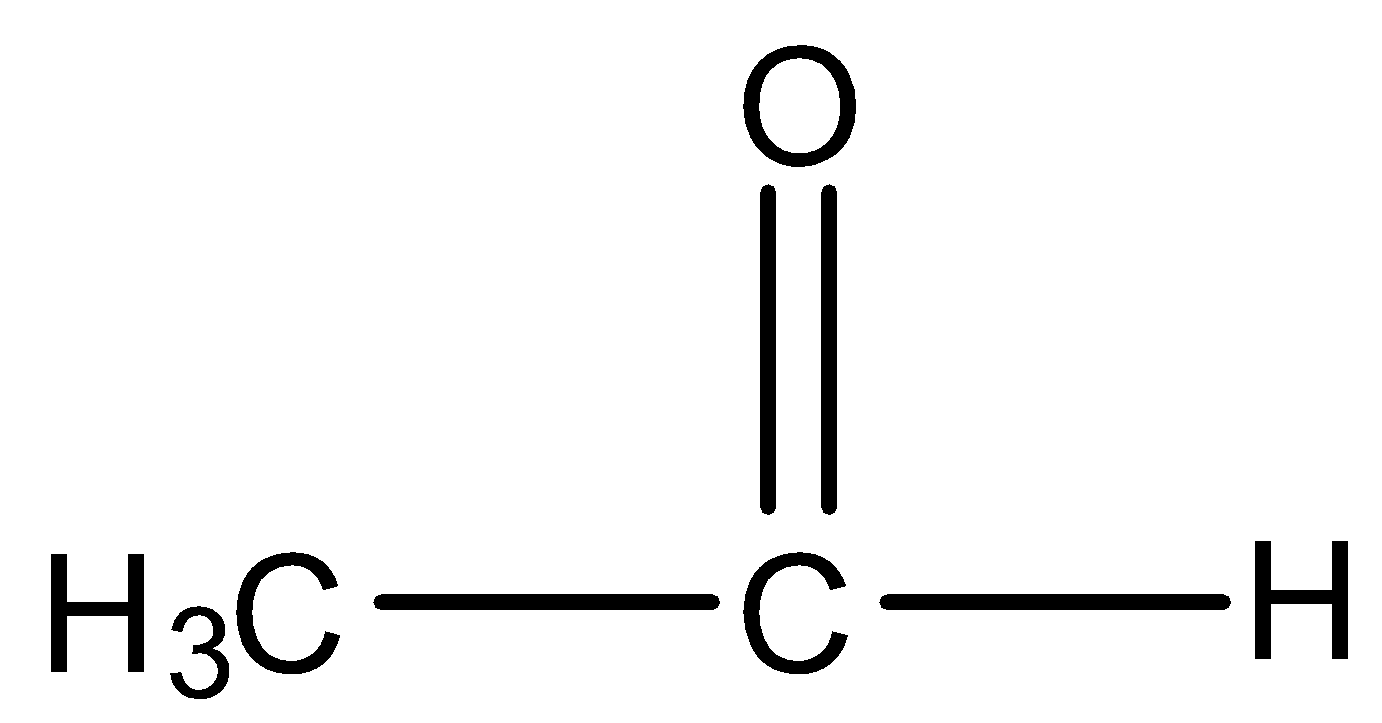
B.
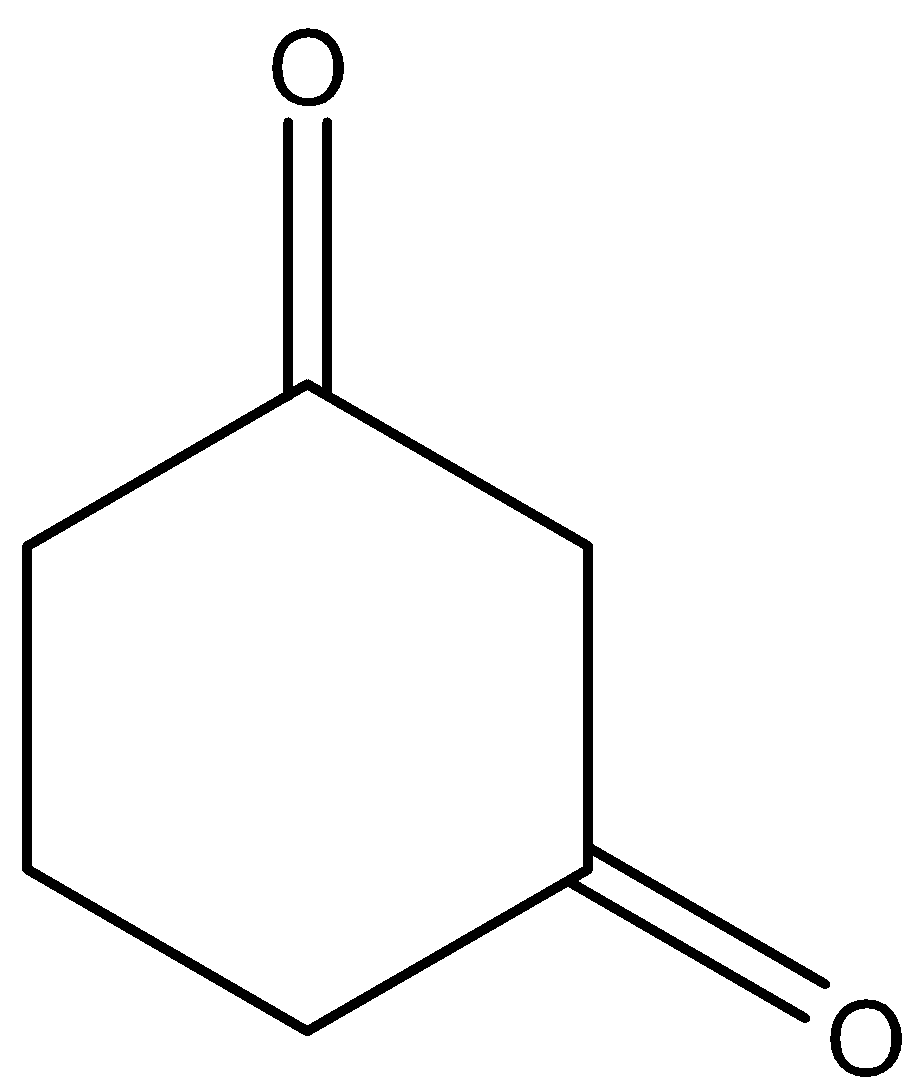
C.
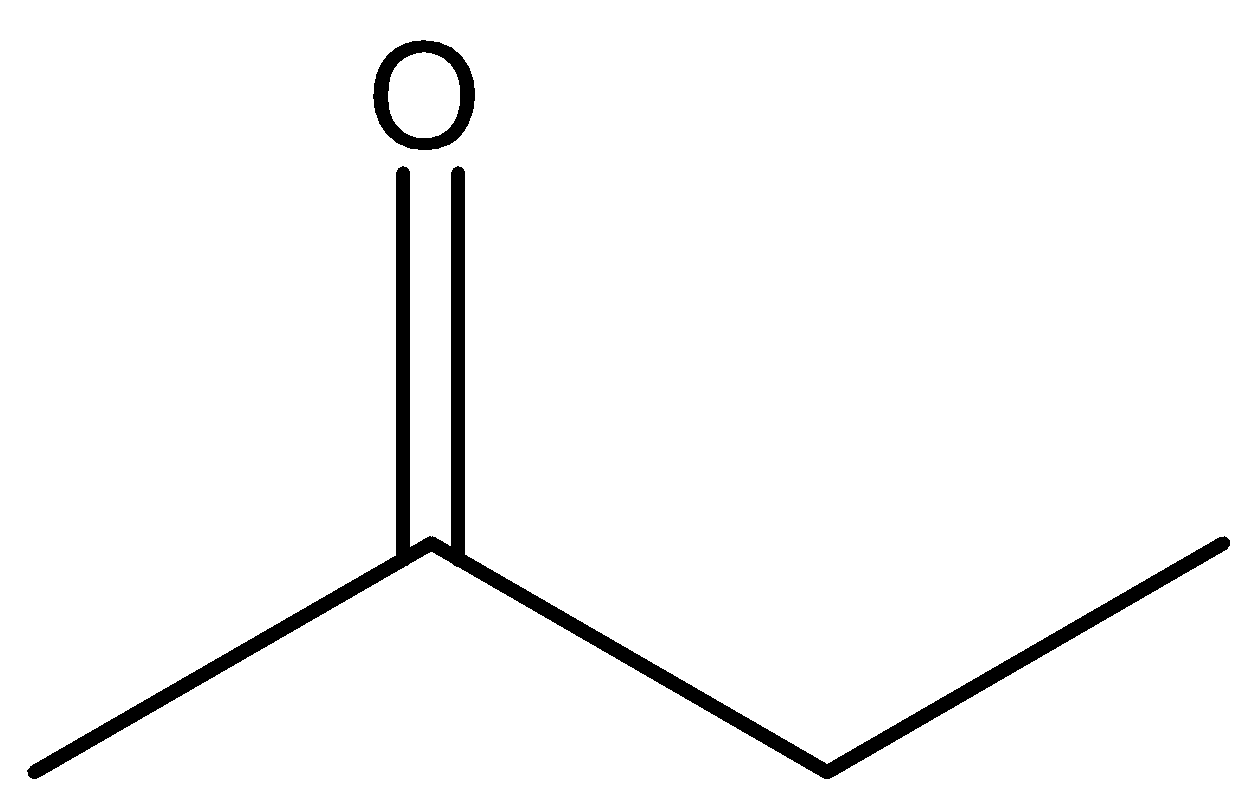
D.All



Answer
522.9k+ views
Hint: $NaOI$ is known as sodium hypo iodide. It is formed by the reaction of \[{I_2}\] and $NaOH$ used in the iodoform reaction. The alcohols, aldehydes and ketones containing \[C{H_3}CO\]group react with $NaOI$ to form yellow precipitate of $CH{I_3}$ which is called as iodoform. This reaction is used as a test for differentiating between different alcohols, aldehydes and ketones.
Complete answer:
If any molecule reacts with $NaOI$ it should have \[C{H_3}CO\] i.e.
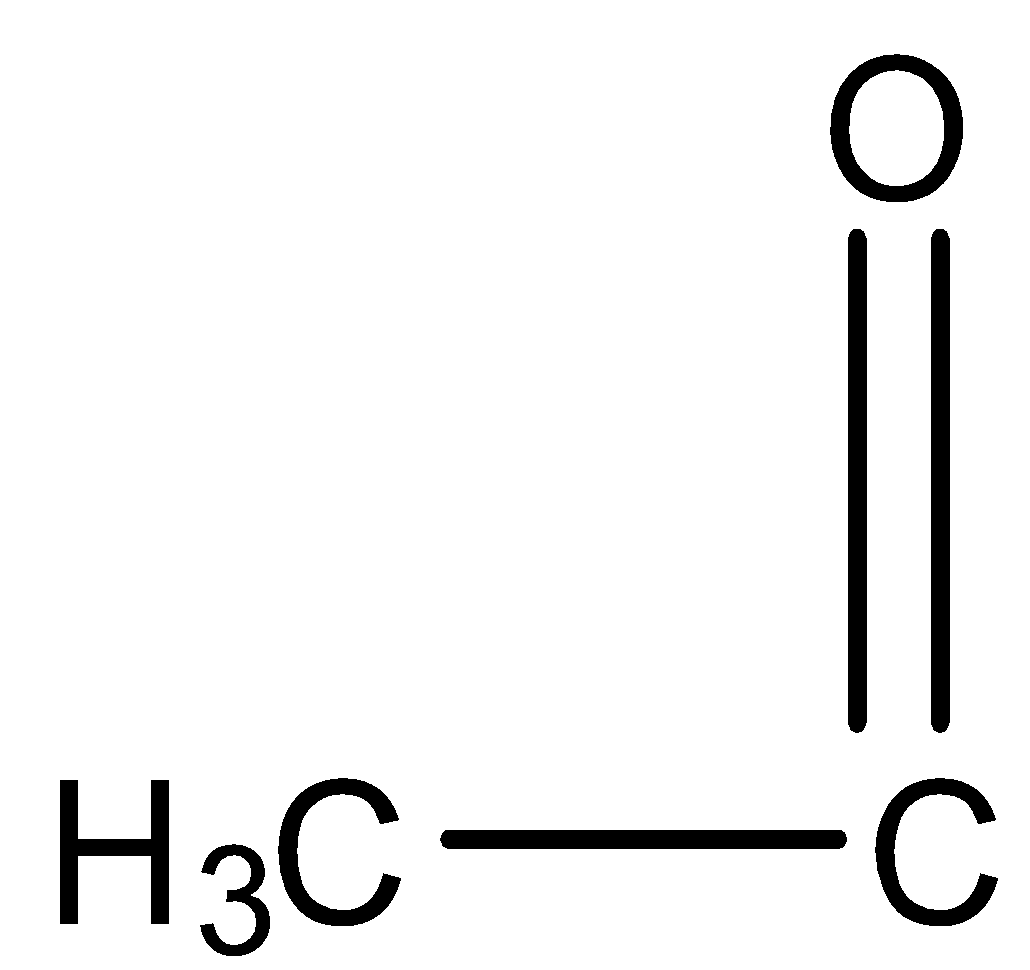 group and at the end of the reaction it will give $CH{I_3}$ as a yellow precipitate. $NaOI$ takes part in a reaction as $NaOH$ and \[{I_2}\].
group and at the end of the reaction it will give $CH{I_3}$ as a yellow precipitate. $NaOI$ takes part in a reaction as $NaOH$ and \[{I_2}\].
First let us look at basic reaction where$NaOI$ will react with
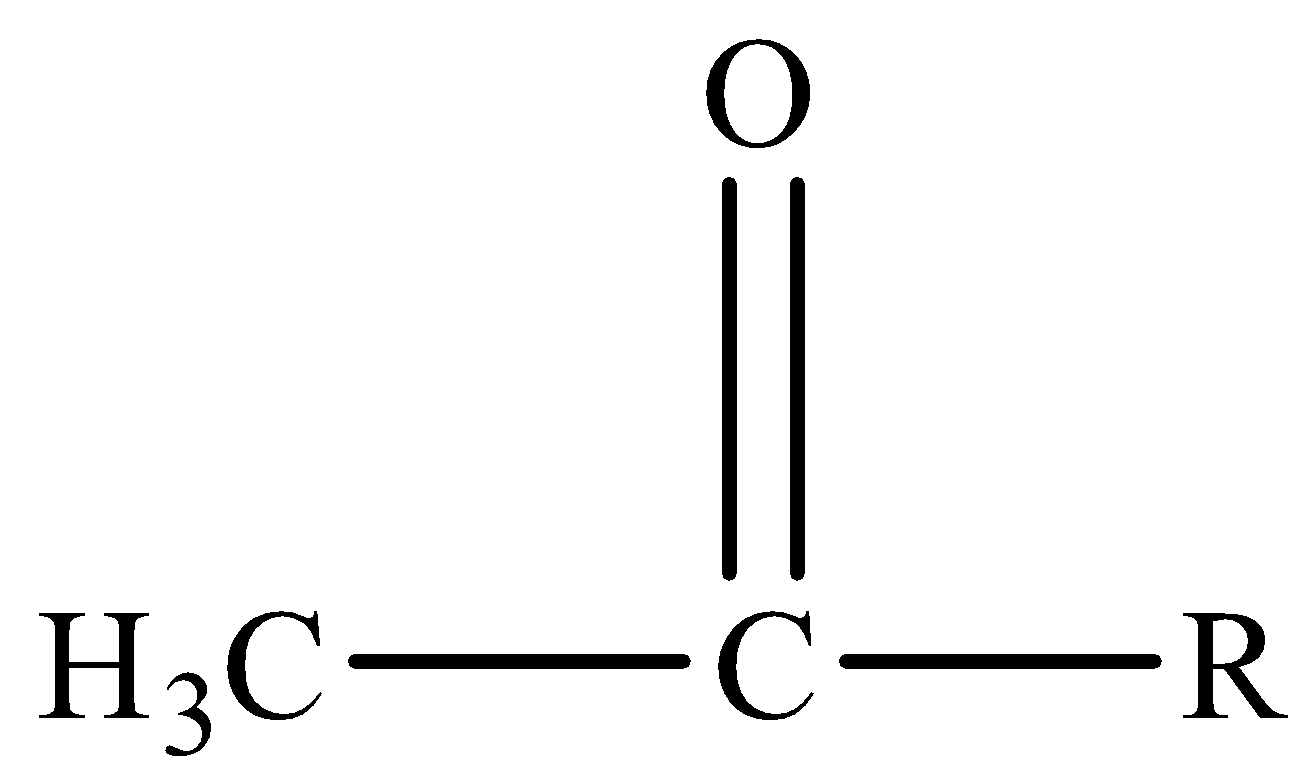 ( where R is any aromatic or aliphatic group)
( where R is any aromatic or aliphatic group)
The mechanism is as follows:
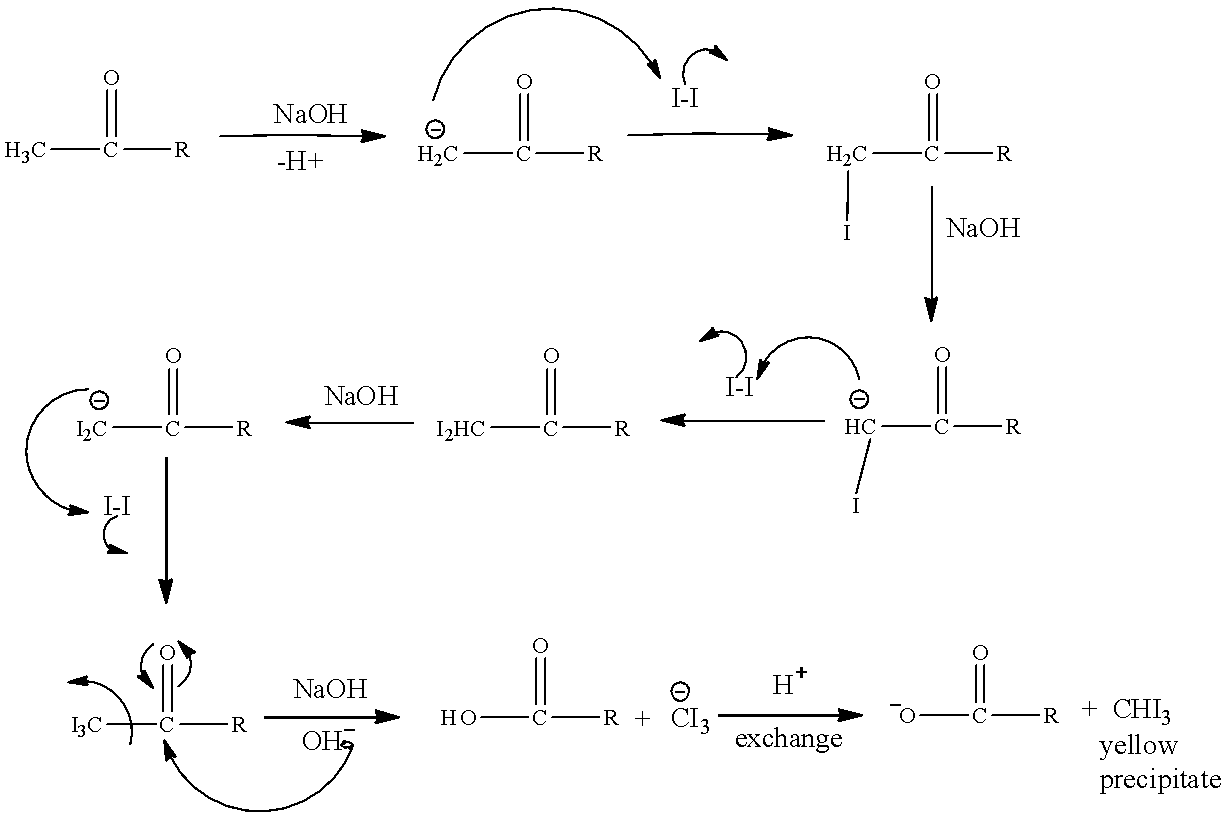
Now we can clearly see in the above mechanism how the \[C{H_3}CO\] group containing molecules is undergoing reaction and in the end $CH{I_3}$ is produced.
So among our options we say that
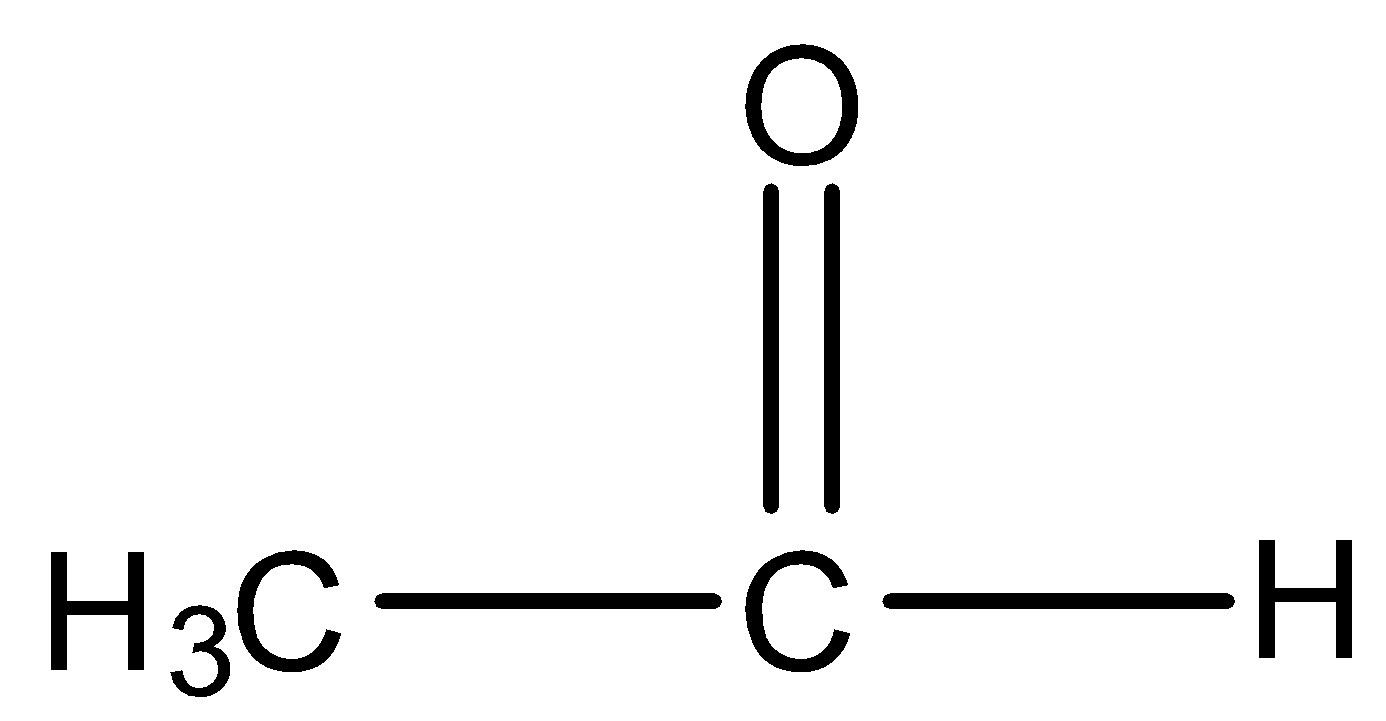 and
and
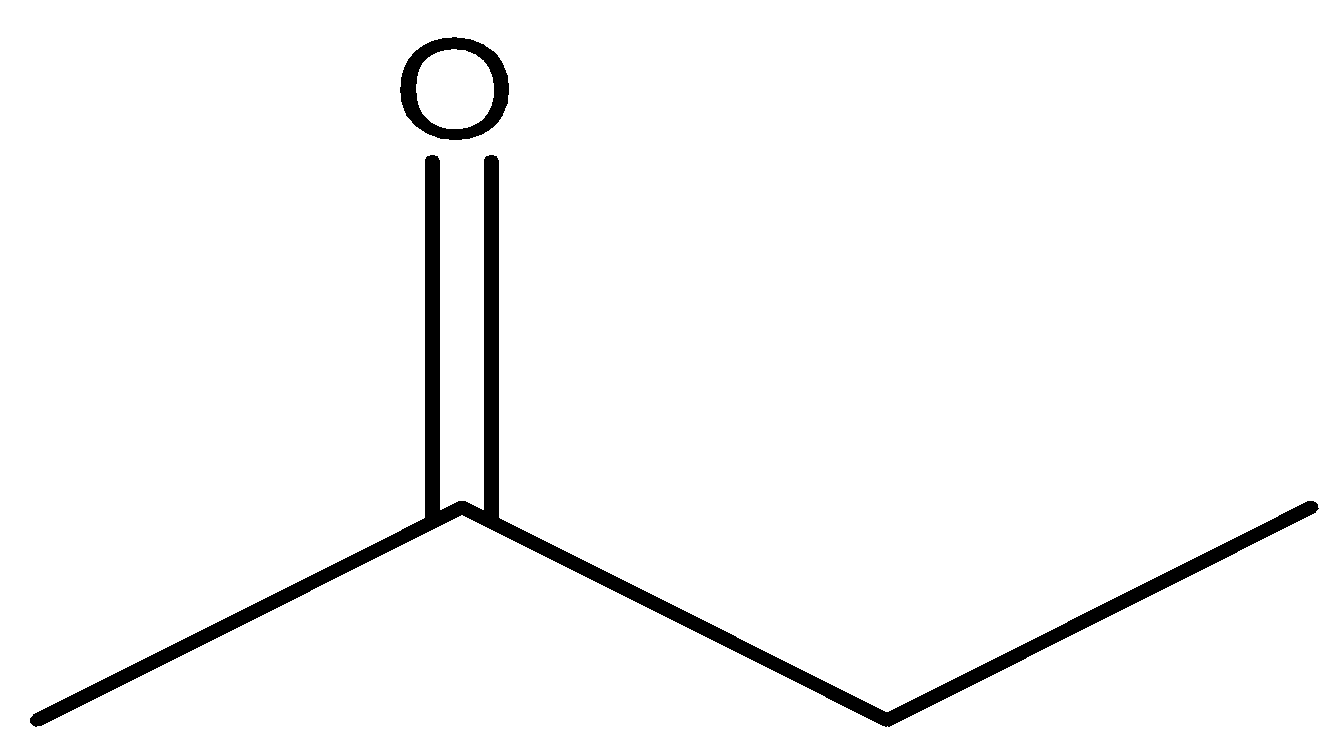 will react with $NaOI$ to give the product as both the molecules contain \[C{H_3}CO\] group in their structure. Now let us look at the aromatic compound.
will react with $NaOI$ to give the product as both the molecules contain \[C{H_3}CO\] group in their structure. Now let us look at the aromatic compound.
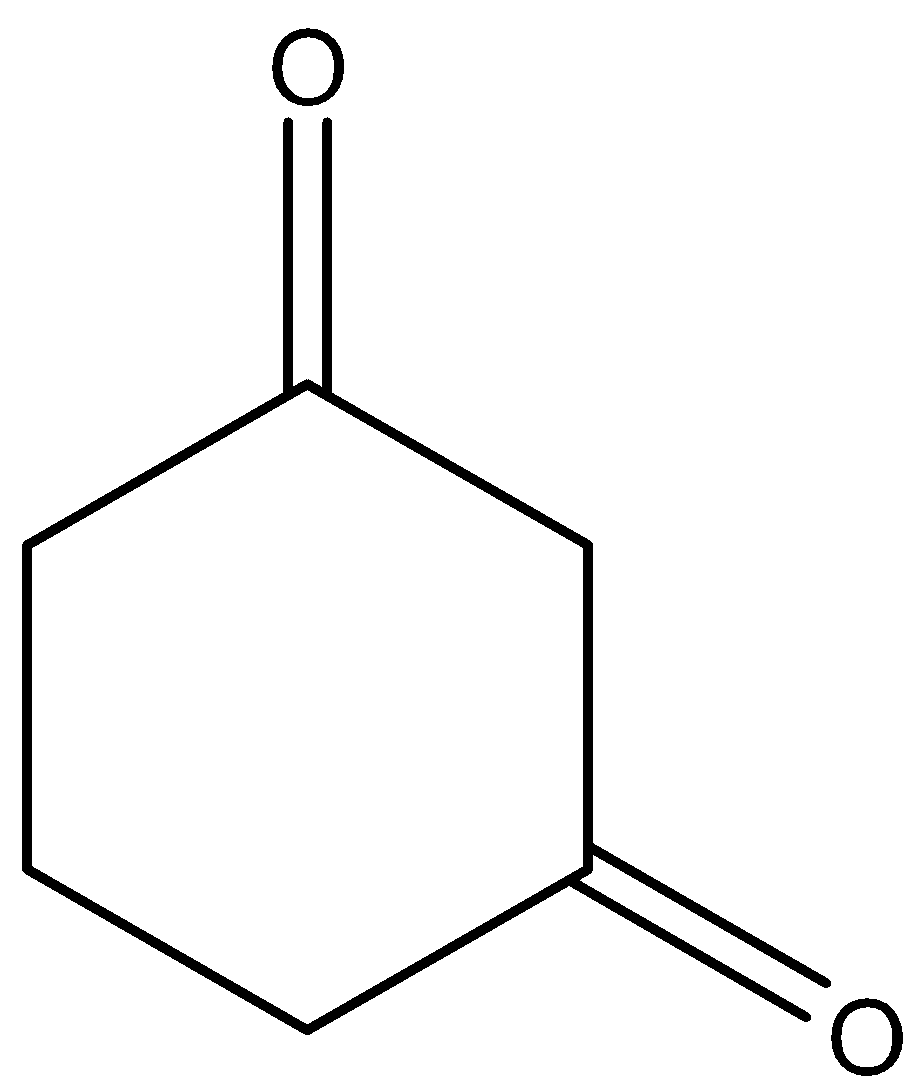 Here this molecule does not have any \[C{H_3}CO\] group in its structure so for knowing whether it will react with $NaOI$ or not we will have to look into its mechanism, which is as follows:
Here this molecule does not have any \[C{H_3}CO\] group in its structure so for knowing whether it will react with $NaOI$ or not we will have to look into its mechanism, which is as follows:
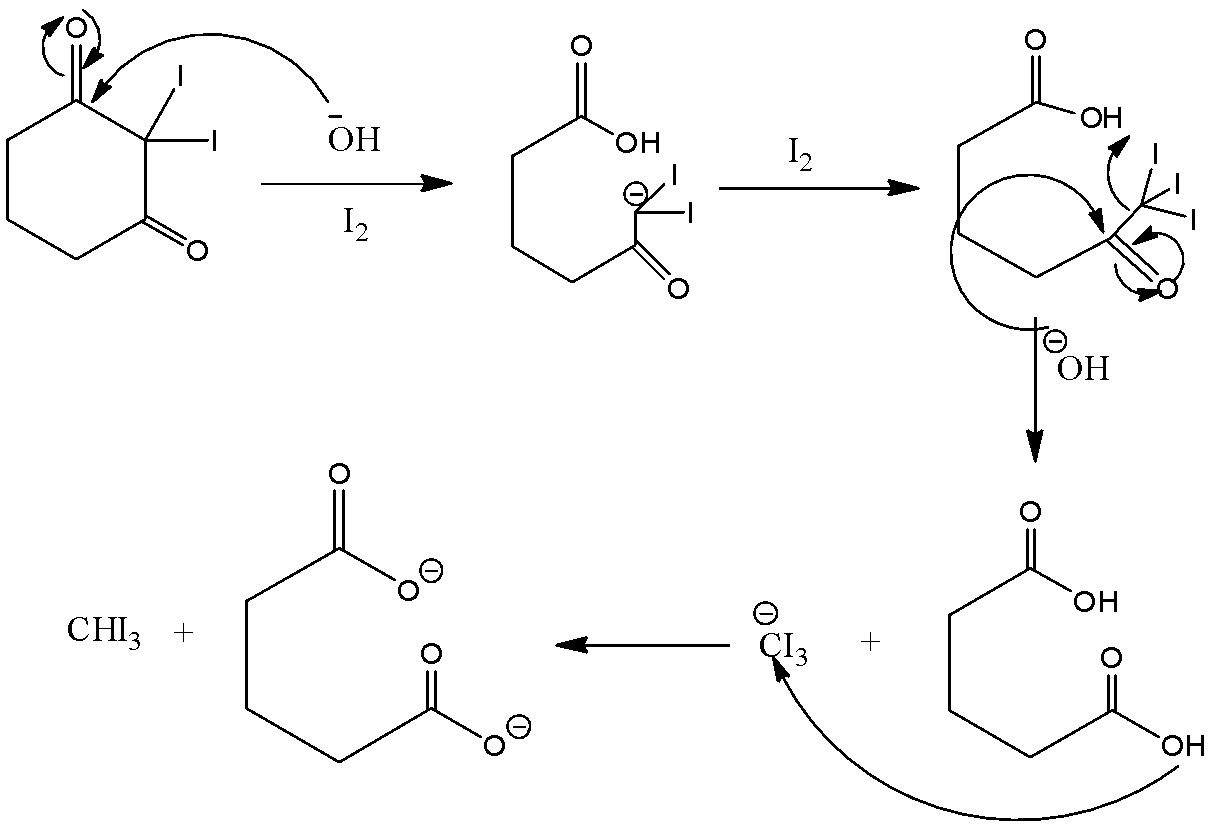
So in the above mechanism base will extract and then $I$ will get attached to it twice, third time there is no \[H\]left so ${}^ - OH$ will then act as nucleophile
We can see that the compound does not have any \[C{H_3}CO\] group but it still reacts with $NaOI$ to give $CH{I_3}$
Therefore all the above molecules will react with $NaOI$.
Hence the correct option is $D.$ All
Note:
Only those molecules will react with $NaOI$ which have\[C{H_3}CO\] group in them and yield $CH{I_3}$ as a precipitate. Even if the molecule does not have\[C{H_3}CO\], in some cases it may react with $NaOI$ and give the yellow precipitate as in the above case.
Complete answer:
If any molecule reacts with $NaOI$ it should have \[C{H_3}CO\] i.e.

First let us look at basic reaction where$NaOI$ will react with

The mechanism is as follows:

Now we can clearly see in the above mechanism how the \[C{H_3}CO\] group containing molecules is undergoing reaction and in the end $CH{I_3}$ is produced.
So among our options we say that




So in the above mechanism base will extract and then $I$ will get attached to it twice, third time there is no \[H\]left so ${}^ - OH$ will then act as nucleophile
We can see that the compound does not have any \[C{H_3}CO\] group but it still reacts with $NaOI$ to give $CH{I_3}$
Therefore all the above molecules will react with $NaOI$.
Hence the correct option is $D.$ All
Note:
Only those molecules will react with $NaOI$ which have\[C{H_3}CO\] group in them and yield $CH{I_3}$ as a precipitate. Even if the molecule does not have\[C{H_3}CO\], in some cases it may react with $NaOI$ and give the yellow precipitate as in the above case.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




