
Which of the following will undergo decarboxylation on heating?
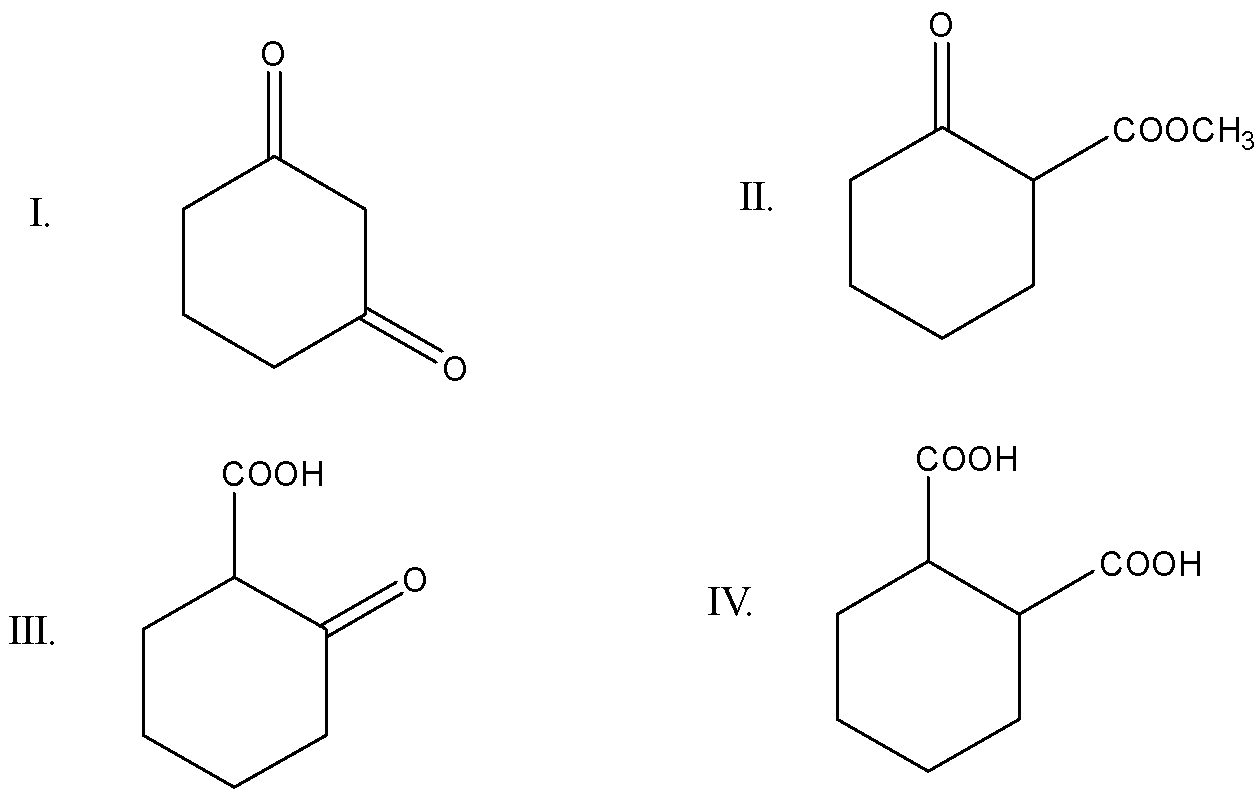
(A) II and III
(B) III and IV
(C) III only
(D) I and IV

Answer
566.7k+ views
Hint: Decarboxylation is usually related to the removal of a carbon atom from the carbon chain of carboxylic acids.So we can look out for the options in which the carbon ring has carboxylic acids attached to it.For decarboxylation to take place, the presence of carboxyl groups is important.
Complete step by step answer:
In option (a), i.e.
1, 3-Diketohexane, there is no carboxyl group present so decarboxylation cannot take place
In option (b) i.e.
There is a carboxyl group present in the form of methyl acetate for carbon dioxide to release, so, decarboxylation can take place
In option (c)
There is a presence of 1 carboxylic acid group along with keto group so, this compound can undergo decarboxylation
In option (d)
2 carboxylic acid groups are present at alpha and beta positions.
Let’s find out which compound amongst these can undergo decarboxylation on heating
Decarboxylation takes place at beta keto acid or beta keto ester. the presence of carboxyl group at the alpha position makes the process of releasing carbon dioxide easier and option (d) cannot be correct as, with only one group of carboxylic acid present, decarboxylation can take place,
So, the correct answer is Option A .
Note: Decarboxylation is the loss of carboxylic acid to release carbon dioxide.Decarboxylation is an important reaction in the mitochondria of the cell to form acetyl coenzyme A. In this reaction, there is reduction of carbon atom. Beta keto acid undergoes decarboxylation very easily.
Complete step by step answer:
In option (a), i.e.
1, 3-Diketohexane, there is no carboxyl group present so decarboxylation cannot take place
In option (b) i.e.
There is a carboxyl group present in the form of methyl acetate for carbon dioxide to release, so, decarboxylation can take place
In option (c)
There is a presence of 1 carboxylic acid group along with keto group so, this compound can undergo decarboxylation
In option (d)
2 carboxylic acid groups are present at alpha and beta positions.
Let’s find out which compound amongst these can undergo decarboxylation on heating
Decarboxylation takes place at beta keto acid or beta keto ester. the presence of carboxyl group at the alpha position makes the process of releasing carbon dioxide easier and option (d) cannot be correct as, with only one group of carboxylic acid present, decarboxylation can take place,
So, the correct answer is Option A .
Note: Decarboxylation is the loss of carboxylic acid to release carbon dioxide.Decarboxylation is an important reaction in the mitochondria of the cell to form acetyl coenzyme A. In this reaction, there is reduction of carbon atom. Beta keto acid undergoes decarboxylation very easily.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




