
Which of the indicated hydrogen is most acidic:
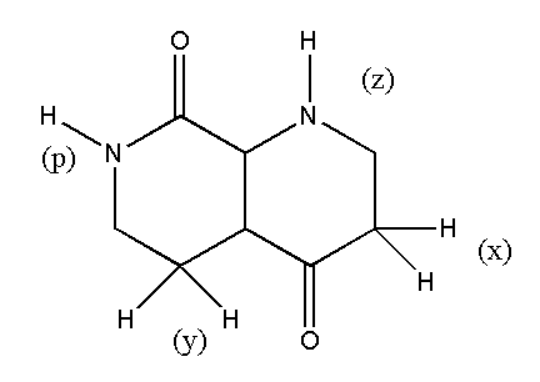
A. (x)
B. (y)
C. (z)
D. (p)

Answer
489.6k+ views
Hint: The acidity of the hydrogen depends on various factors. Few of them are the stability of hydrogen and the electronegativity of the atom it is attached to. The stability of the structure can be due to conjugation or inductive effect. We will consider all the parameters for finding the most acidic hydrogen in the compound.
Complete Step By Step Answer:
The acidity of hydrogen depends on some of the factors. Few of them are like the stability of the compound and the electronegativity of the atom to which hydrogen is attached. From the above structure we can observe that the most electronegative atom to which hydrogen is attached is nitrogen. Therefore we can say that we have to find the more acidic hydrogen between p and z.
Now let us assume that (p) hydrogen is removed and then the stability of compound can be observed as:
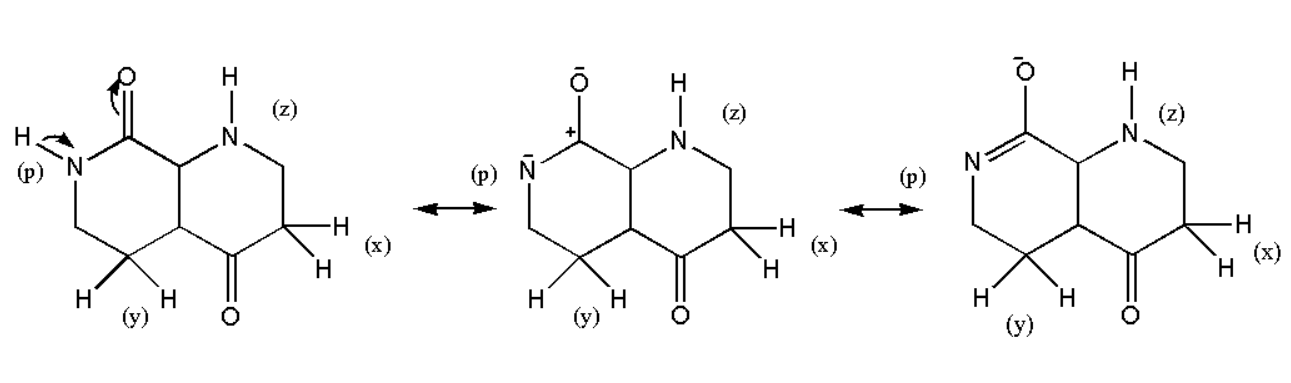
Therefore the compound will be stabilized by resonance. On the other if a hydrogen (z) is removed then we have the following structure:
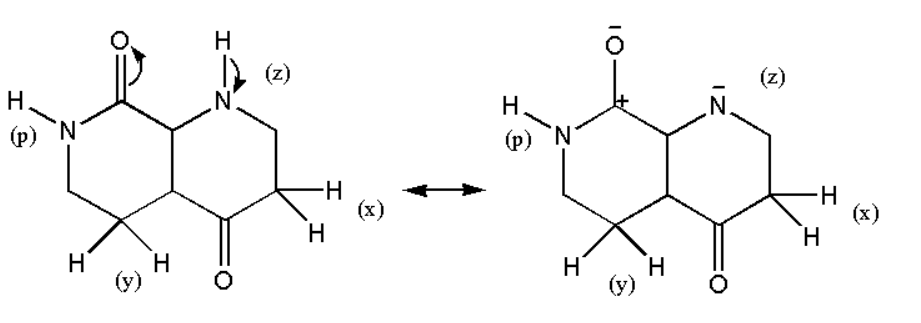
Thus it is not stabilized by resonance and hence we can say that the hydrogen (z) will not be acidic more than that of (p).
Thus we can say that the most stabilized structure is obtained when hydrogen (p) is removed thus it is the most acidic hydrogen.
Hence the correct option is D. (p).
Note:
When hydrogen leaves its position then negative charge is developed on the atom on which it is attached. Thus the electronegative element has a higher tendency to gain negative charge. Thus more electronegative atoms having hydrogen will make it acidic. The negative charge will be more stable on electronegative elements than electropositive elements. More the delocalization of electrons more will be the acidity of hydrogen.
Complete Step By Step Answer:
The acidity of hydrogen depends on some of the factors. Few of them are like the stability of the compound and the electronegativity of the atom to which hydrogen is attached. From the above structure we can observe that the most electronegative atom to which hydrogen is attached is nitrogen. Therefore we can say that we have to find the more acidic hydrogen between p and z.
Now let us assume that (p) hydrogen is removed and then the stability of compound can be observed as:

Therefore the compound will be stabilized by resonance. On the other if a hydrogen (z) is removed then we have the following structure:

Thus it is not stabilized by resonance and hence we can say that the hydrogen (z) will not be acidic more than that of (p).
Thus we can say that the most stabilized structure is obtained when hydrogen (p) is removed thus it is the most acidic hydrogen.
Hence the correct option is D. (p).
Note:
When hydrogen leaves its position then negative charge is developed on the atom on which it is attached. Thus the electronegative element has a higher tendency to gain negative charge. Thus more electronegative atoms having hydrogen will make it acidic. The negative charge will be more stable on electronegative elements than electropositive elements. More the delocalization of electrons more will be the acidity of hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




