
Which of these are respiratory poisons or inhibitors of ETC?
a. Cyanides
b. Antimycin A
c. Carbon monoxide
d. All of these
Answer
580.5k+ views
Hint: Electron transport chain is composed of a series of complexes which are known for transferring electrons from an electron donor to electron acceptors through a redox reaction. So, the inhibitors of ETC will inhibit this process of electron transfer.
Complete answer:
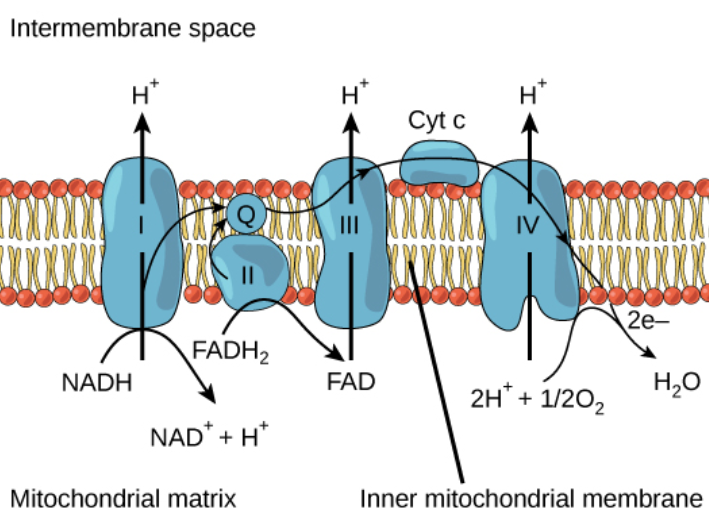
Electron Transport Chain (ETC) refers to a series of complexes that are known for transferring electrons to generate ATP. The inhibition of ETC will result in the accumulation of oxidized forms of the components of ETC after the point of inhibition and the reduced forms before the point of inhibition. This inhibition will tend to stop the production of ATP. The most important inhibitors of ETC are amytal, rotenone, antimycin A, cyanides, sodium azide, and CO.
Amytal and rotenone are known to block the ETC between NADH dehydrogenase (Complex I) and CoQ.
Cyanides, sodium azides, and carbon monoxide (CO) are known for inhibiting the electron transport chain by binding to \[F{e^{ + 3}}\] in Haem groups in cytochrome oxidase. Thereby, inhibiting the terminal transfer of electrons to oxygen.
Antimycin A is an antibiotic that inhibits the ETC by interfering with the electron flow at the level of $CoQ{H_2}$-cytochrome c reductase (complex III).
Hence, the correct answer is option (D).
Additional information:
Electron transport chain is located in the inner mitochondrial membrane and it is the final stage of aerobic respiration. The ETC is known to synthesize the energy in the form of ATP by a process called oxidative phosphorylation because the energy that is required to produce ATP is derived from the oxidation of hydrogen carriers.
Note: The inhibitors of ETC are those substances that bind to the components of ETC thereby blocking the transfer of electrons and hence, preventing its ability to change in its reversible form i.e., from oxidized state to reduced state.
Complete answer:
Electron Transport Chain (ETC) refers to a series of complexes that are known for transferring electrons to generate ATP. The inhibition of ETC will result in the accumulation of oxidized forms of the components of ETC after the point of inhibition and the reduced forms before the point of inhibition. This inhibition will tend to stop the production of ATP. The most important inhibitors of ETC are amytal, rotenone, antimycin A, cyanides, sodium azide, and CO.
Amytal and rotenone are known to block the ETC between NADH dehydrogenase (Complex I) and CoQ.
Cyanides, sodium azides, and carbon monoxide (CO) are known for inhibiting the electron transport chain by binding to \[F{e^{ + 3}}\] in Haem groups in cytochrome oxidase. Thereby, inhibiting the terminal transfer of electrons to oxygen.
Antimycin A is an antibiotic that inhibits the ETC by interfering with the electron flow at the level of $CoQ{H_2}$-cytochrome c reductase (complex III).

Hence, the correct answer is option (D).
Additional information:
Electron transport chain is located in the inner mitochondrial membrane and it is the final stage of aerobic respiration. The ETC is known to synthesize the energy in the form of ATP by a process called oxidative phosphorylation because the energy that is required to produce ATP is derived from the oxidation of hydrogen carriers.
Note: The inhibitors of ETC are those substances that bind to the components of ETC thereby blocking the transfer of electrons and hence, preventing its ability to change in its reversible form i.e., from oxidized state to reduced state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




