
Which one of the following does not have $s{p^2}$ hybridised carbon?
A.Acetone
B.Acetic Acid
C.Acetonitrile
D.Acetamide
Answer
553.8k+ views
Hint: In this question, we need to determine that of the given options which compound does not have $s{p^2}$ hybridised carbon. For this, we will first draw the structure of each of the given options and then back bonding form. Hybridisation can easily be recognized by seeing the structure of the compound.
Complete step-by-step answer:
Let’s see the (a) acetone structure,
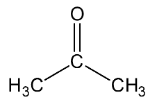
In \[C = O\], the C is \[s{p^2}\] hybridised.
In both \[C{H_3}\], the\[C - H\] single bound therefore the C is \[s{p^3}\] hybridised.
Let’s see the (b) acetic acid structure,
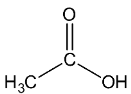
In \[C = O\], the C is \[s{p^2}\] hybridised.
In both \[C{H_3}\], the \[C - H\] single bound therefore the C is \[s{p^3}\] hybridised.
Let’s see the (c) acetonitrile structure,
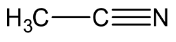
In \[C \equiv N\], the C is \[sp\] hybridised .
In both \[C{H_3}\], the \[C - H\] single bound therefore the C is \[s{p^3}\] hybridised.
Let’s see the (d) acetamide structure,
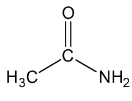
In \[C = O\], the C is \[s{p^2}\] hybridised .
In both \[C{H_3}\], the \[C - H\] single bound therefore the C is \[s{p^3}\] hybridised .
Therefore, (C) is the correct answer. Because the (c) option doesn’t have \[s{p^2}\] hybridised Carbon.
Note: Remember to draw structures and make sure that common names and IUPAC names should be known. Nitrogen can form triple and in ionic form it forms four bonds. In this question the acetonitrile was not showing any \[s{p^2}\] hybridised Carbon because of the structure. \[s{p^2}\] carbon shows \[one\;\sigma - two\;\pi \] bonds, whereas in acetonitrile’s case we are able to observe that it shows \[sp\] hybridised \[one\;\sigma - two\;\pi \] bonds.
Complete step-by-step answer:
Let’s see the (a) acetone structure,

In \[C = O\], the C is \[s{p^2}\] hybridised.
In both \[C{H_3}\], the\[C - H\] single bound therefore the C is \[s{p^3}\] hybridised.
Let’s see the (b) acetic acid structure,

In \[C = O\], the C is \[s{p^2}\] hybridised.
In both \[C{H_3}\], the \[C - H\] single bound therefore the C is \[s{p^3}\] hybridised.
Let’s see the (c) acetonitrile structure,
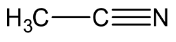
In \[C \equiv N\], the C is \[sp\] hybridised .
In both \[C{H_3}\], the \[C - H\] single bound therefore the C is \[s{p^3}\] hybridised.
Let’s see the (d) acetamide structure,

In \[C = O\], the C is \[s{p^2}\] hybridised .
In both \[C{H_3}\], the \[C - H\] single bound therefore the C is \[s{p^3}\] hybridised .
Therefore, (C) is the correct answer. Because the (c) option doesn’t have \[s{p^2}\] hybridised Carbon.
Note: Remember to draw structures and make sure that common names and IUPAC names should be known. Nitrogen can form triple and in ionic form it forms four bonds. In this question the acetonitrile was not showing any \[s{p^2}\] hybridised Carbon because of the structure. \[s{p^2}\] carbon shows \[one\;\sigma - two\;\pi \] bonds, whereas in acetonitrile’s case we are able to observe that it shows \[sp\] hybridised \[one\;\sigma - two\;\pi \] bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




