
Which one of the following is an elastomer?
A.
B.
C.
D.




Answer
515.1k+ views
Hint: The polymers which are held together by weak intermolecular forces and have a tendency to regain its shape after and size after stretching it to a greater extent are called elastomers. These are composed of long chain-like molecules bonded via covalent bonding.
Complete answer:
The given polymers are explained on the basis of their properties as follows:
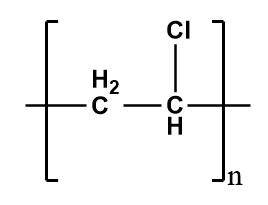
Polyvinyl chloride (PVC)-
It is a thermoplastic polymer i.e.; it is moldable with a certain rise in temperature and solidifies on cooling. They have relatively weak intermolecular forces which results in the softening of the material. If the temperature is raised too high, it results in the irreversible degradation of the material but on lowering the temperature, it will return to its solid phase by regaining its shape. Therefore, it shows the elastic properties and thus, is an example of an elastomer.
The structure of PVC is as follows-
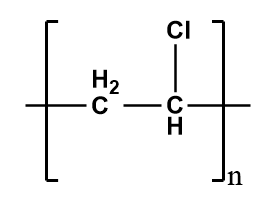
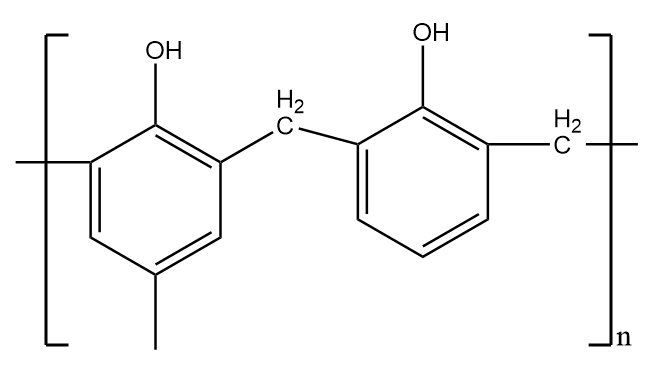
Bakelite-
It is a thermosetting polymer i.e.; they form a permanent shape on heating through an irreversible process and cannot regain its shape on lowering the temperature. These structures are formed by covalent bonds with relatively higher energy and do not easily break when heated. Therefore, it does not show elastic properties and thus, is not an example of an elastomer.
The structure of Bakelite is as follows:
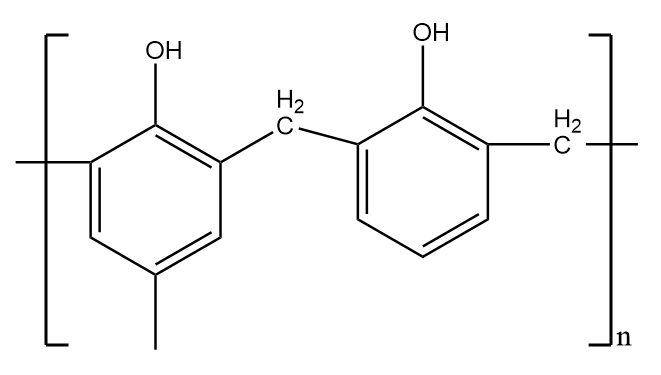
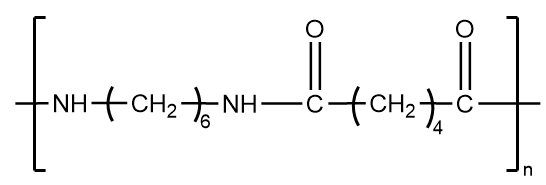
Nylon 6,6-
It is an example of fiber which consists of thread-like structure with high tensile strength and high value of young modulus. Because the structure is held together by strong intermolecular forces, it does not have a tendency to show elastic properties and hence, is not an example of an elastomer.
The structure of nylon 6,6 is as follows:
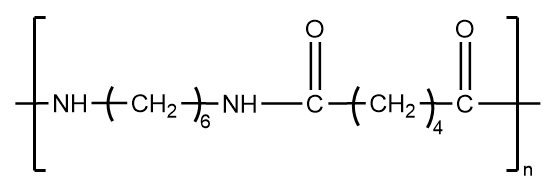
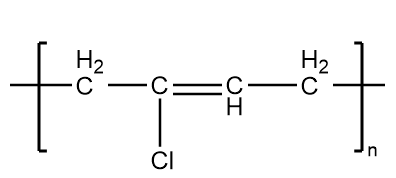
Neoprene-
It is an example of synthetic rubber which is formed on the polymerization reaction of chloroprene. It has good chemical stability and sustain flexibility over a broad range of temperatures. Therefore, it shows the elastic properties and thus, is an example of an elastomer.
The structure of neoprene is as follows:
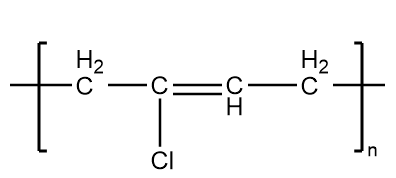
Hence, options (A) and (D) are the correct answer.
Note:
Ensure not to get confused between the terms elastomer and plastic as they are not similar to each other. The molecular characteristics overlap for both plastic and elastomers but they are defined by their response to temperature, chemical reactions and mechanical stress.
Complete answer:
The given polymers are explained on the basis of their properties as follows:
Polyvinyl chloride (PVC)-
It is a thermoplastic polymer i.e.; it is moldable with a certain rise in temperature and solidifies on cooling. They have relatively weak intermolecular forces which results in the softening of the material. If the temperature is raised too high, it results in the irreversible degradation of the material but on lowering the temperature, it will return to its solid phase by regaining its shape. Therefore, it shows the elastic properties and thus, is an example of an elastomer.
The structure of PVC is as follows-

Bakelite-
It is a thermosetting polymer i.e.; they form a permanent shape on heating through an irreversible process and cannot regain its shape on lowering the temperature. These structures are formed by covalent bonds with relatively higher energy and do not easily break when heated. Therefore, it does not show elastic properties and thus, is not an example of an elastomer.
The structure of Bakelite is as follows:

Nylon 6,6-
It is an example of fiber which consists of thread-like structure with high tensile strength and high value of young modulus. Because the structure is held together by strong intermolecular forces, it does not have a tendency to show elastic properties and hence, is not an example of an elastomer.
The structure of nylon 6,6 is as follows:

Neoprene-
It is an example of synthetic rubber which is formed on the polymerization reaction of chloroprene. It has good chemical stability and sustain flexibility over a broad range of temperatures. Therefore, it shows the elastic properties and thus, is an example of an elastomer.
The structure of neoprene is as follows:

Hence, options (A) and (D) are the correct answer.
Note:
Ensure not to get confused between the terms elastomer and plastic as they are not similar to each other. The molecular characteristics overlap for both plastic and elastomers but they are defined by their response to temperature, chemical reactions and mechanical stress.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




